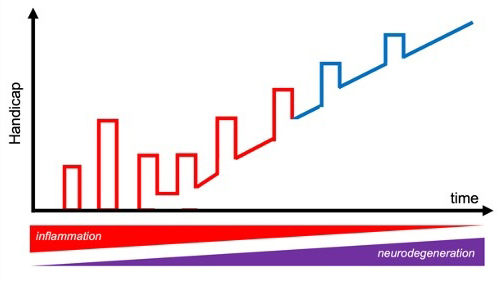
Multiple sclerosis, or MS, is a treacherous disease: This disorder of the central nervous system, in which the immune system attacks the protective sheath that covers nerve fibers, progresses in cycles of remission and relapse. That is, the body repeatedly manages to recover from the attack and keep the disease in check, before eventually succumbing to it.
Extending the remissions and delaying the flare-ups can buy valuable time for people with MS. Achieving this is possible, temporarily at least, with some drugs, including Copaxone®, which was developed at the Weizmann Institute of Science. But understanding the underlying mechanism may hold the key to beating the disease altogether. Yet, relatively little is known about what causes the remissions – or how to induce them.
In a recent study, a team of Weizmann Institute researchers has made a significant step toward identifying the cause of remission. The researchers, led by Prof. Steffen Jung of the Immunology and Regenerative Biology Department, identified “master regulators” of remission: brain immune cells called microglia. These help keep in check the attacking agents that drive the disorder, which are a different kind of immune cell. “Our basic research in the animal model is aimed at both understanding how the pathology progresses – how the body deals with the attack – and identifying the important players, but it may eventually lead to developing targeted therapies, which we currently lack,” Jung says.

In MS, Jung explains, nerve sheaths come under attack from the immune system’s T cells. The assaulting T cells, called effectors, mistake the sheath’s molecules for those of an infectious microorganism or other danger. Another type of T cell, known as a regulatory T cell, inhibits the effectors; when it fails to do so, the disease flares up. The balance of these opposing activities determines the disease outcome.
""To understand these intricacies of the body is rewarding in itself, but now, for clinicians looking for ways to manipulate elements of the immune system to beat MS, these cells and molecules could be potential targets"
But neither effector nor regulatory T cells act alone. They generally need instructions from other cells, which “chew up” appropriate targets for the T cell response and display chunks of those targets, known as antigens, to the T cells. It was the identity of such antigen “presenters” in MS pathology that was unknown. Jung’s team set out to investigate their prime candidates, the microglia. Microglia are a type of macrophage residing in the brain. These cells clear debris but also have many other roles, including the pruning of neuronal synapses and other functions specific to the central nervous system.
Knowing When to Look
Initial experiments, however, did not support Jung’s hypothesis about microglia’s involvement in MS. His team deleted surface molecules for antigen presentation from the microglia in mice and then induced in these animals a disease that is analogous to MS in humans.
“The result was disappointing – mice with edited microglia got sick in just the same way as mice with unaltered microglia,” Jung said about those experiments. “It suggested that microglia weren’t important for the onset of MS.”
Or, the team thought, is it possible that the microglia kick into action after MS has already set in?
Of MS, Mice and Men
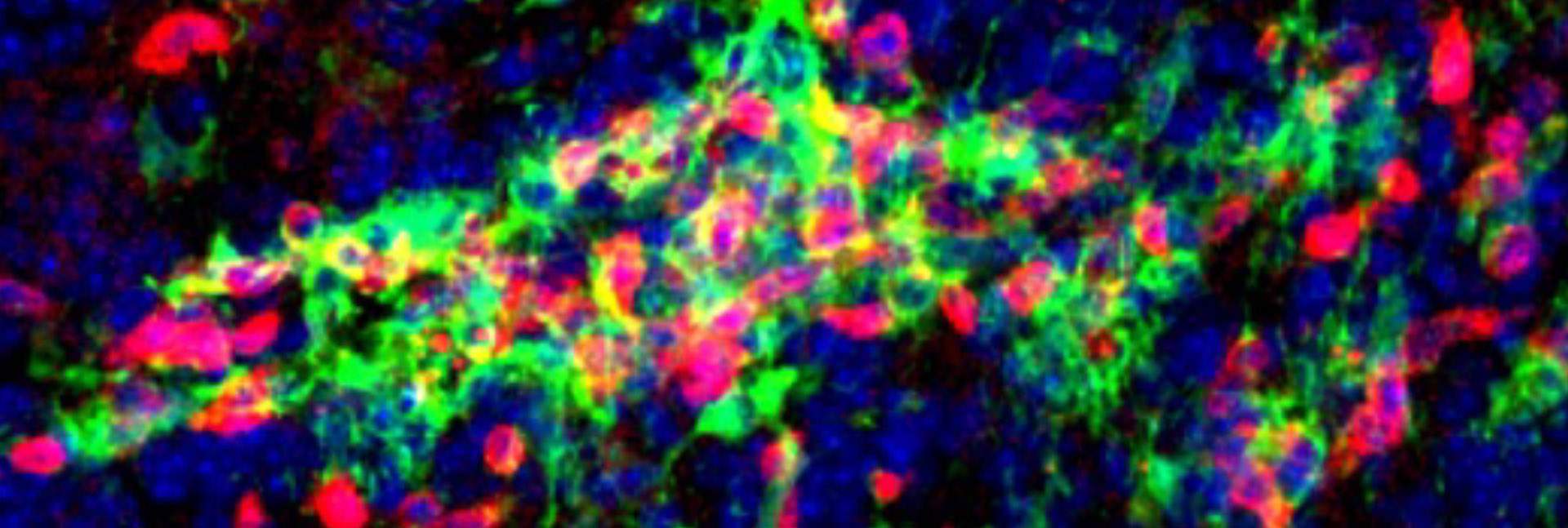
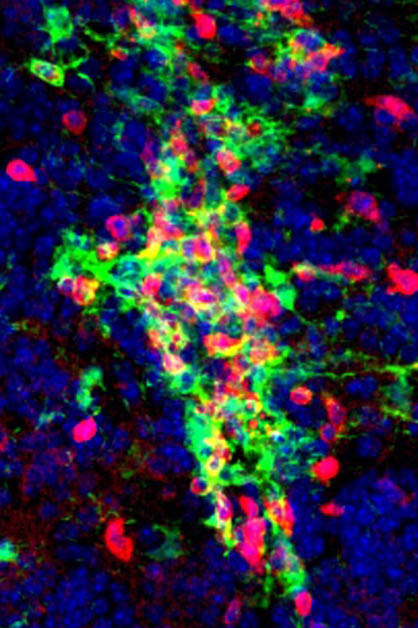
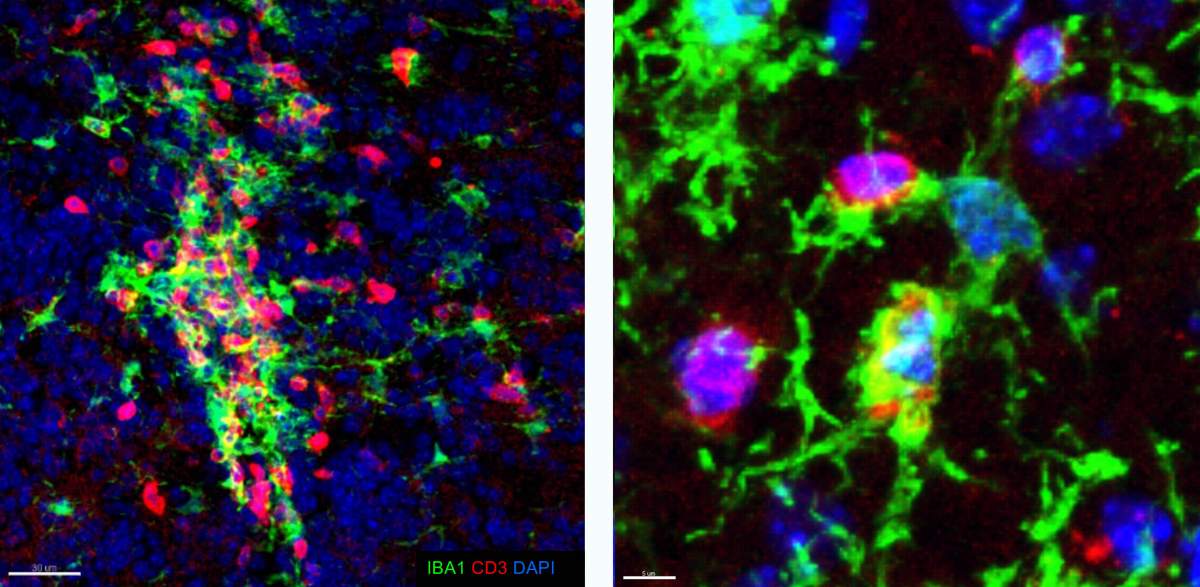
To find out, Jung’s team began experimenting with a new strain of laboratory mice in which the MS-like disease progresses through remission and relapse, just as in humans. This model allowed the researchers to show that during the pathology, microglia expressed genes known to be important for interactions with T cells, and that they even tended to cluster together with the calming, regulatory T cells.
Collectively, these findings suggested that microglia might indeed play a role in MS remission, but the clustering, which can occur in a number of situations, did not explain the mechanism or whether it was significant.
So Jung’s team repeated their original experiment. They deleted the antigen-presenting molecules from the microglia as before, as well as another receptor that is essential for interaction with T cells. The results were clearcut: “The mice with the altered microglia developed the MS-like disease, but it ran a chronic, constant course – without remission,” Jung says. “This strongly suggests that microglia are responsible for remission caused by regulatory T cells.”
The scientists noted that the number of interactions between microglia and regulatory T cells was decreased in the mice with altered microglia. In fact, many of the regulatory T cells underwent a change, becoming similar to the disease-causing T effector cells.