Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Our reflection in the mirror informs our internal perception of our size – whether we believe ourselves to be tall or short, slim or full-bodied, lanky or chubby – and this perception in turn affects how we behave. The cells in our bodies must assess their size, as well, so that they can adjust their metabolic rates to their individual cellular sizes; but how this adjustment works over an entire range of dimensions has been a mystery. Neurons, in particular, come in a staggering variety of sizes: In large mammals, the length of their extensions ranges from a few microns (millionths of a meter) in the brain to more than a meter in the legs.

A few years ago, Prof. Mike Fainzilber and his team in Weizmann Institute’s Biomolecular Sciences Department discovered a sensing mechanism by which nerve cells assess their dimensions and direct their growth. It consists of molecular tracks on which signals travel like railcars from the cell’s center to its periphery, and back. The frequency of the signaling is what helps the cell get a sense of its dimensions: “Railcars” that rapidly bounce off the remote edges and return to the center suggest a smaller cell size; those that take longer to bounce back and are therefore less frequent, suggest bigger ones.
Now in a new study published in Cell Reports, postdoctoral fellow Dr. Rotem Ben-Tov Perry, staff scientist Dr. Ida Rishal and research student Ella Doron-Mandel, together with other members of Fainzilber’s team and collaborators, reveal the molecular machinery that sets this mechanism in motion. First, the scientists identified a crucial size-sensing railcar, a protein called importin-beta1. It works as follows: An mRNA molecule encoding the genetic instructions for making importin-beta1 rides on the molecular tracks from the cell center. When this mRNA reaches the end of the tracks, the importin-beta1 protein is synthesized, and this protein then delivers the sensing signal back to the cell nucleus.
Neurons, in particular, come in a staggering variety of sizes
Next, seeking to discover how the mRNA of importin-beta1 is transported along the molecular tracks, the scientists resorted to somewhat unusual source material. From a slaughterhouse in northern Israel, they obtained bovine sciatic nerves, which are normally removed and discarded because their consumption is prohibited under the Jewish dietary laws of Kashrut. Neurons in the sciatic nerve possess some of the longest extensions in the body; their size helped the researchers to identify a key protein, nucleolin, which binds to the mRNA of importin-beta1. This, in turn, prompts the nucleolin to travel together with this mRNA – like a two-railcar train – from the cell’s nucleus to its periphery. When the scientists disrupted the activity of nucleolin using an experimental anti-cancer drug that binds to this protein, both railcars – nucleolin and the mRNA of importin-beta1, stayed in the cell center, traveling only a short distance toward the periphery, if at all.
Because the distance traveled was so short, the “traffic frequency” on the size-sensing molecular tracks increased substantially, “fooling” the neuron into thinking its extension was much smaller than it actually was. The neuron responded by growing at up to five times the usual rate, ending up with an extension that was much longer than usual. Using the same genetic manipulations, the scientists were able to trick not only neurons but also connective tissue cells into growing larger than normal.
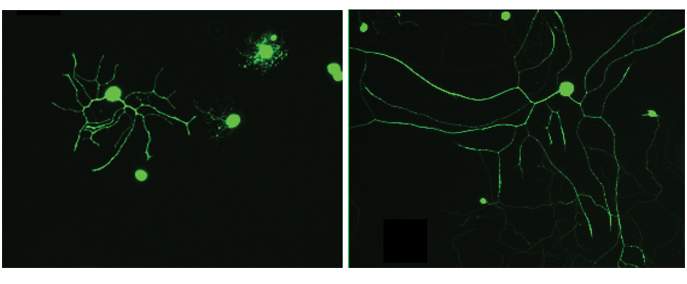
These findings may have implications for cancer research and nerve regeneration. Nucleolin is found in large amounts in certain cancer cells, and the experimental nucleolin-binding anti-cancer drug used in the study kills these cells, but the drug’s precise mechanism of action is unknown. The Weizmann study, suggesting that nucleolin is part of a size-sensing mechanism that regulates cellular growth, may open up a new direction in the search for improved anti-cancer medications. Moreover, an in-depth understanding of the size-sensing mechanism that regulates the growth of neurons may in the future help develop the means of speeding up nerve regeneration after injury.
Also taking part in the study were Dr. Marco Terenzio, Stefanie Alber, Dr. Sandip Koley, Albina Lin, Dr. Meir Rozenbaum, Dr. Dmitry Yudin and Prof. Avraham Yaron of Weizmann’s Biomolecular Sciences Department; Dr. Ashley L. Kalinski, Dr. Pabitra K. Sahoo, Dr. Cynthia Gomes and Prof. Jeffery L. Twiss of the University of South Carolina; Dr. Katalin F. Medzihradszky and Prof. Alma L. Burlingame of the University of California, San Francisco; Dr. Vera Shinder of Weizmann’s Chemical Research Support Department; Dr. Wasim Geraisy of Tnuva; and Dr. Eric A. Huebner and Prof. Clifford J. Woolf of Harvard Medical School.
Prof. Michael Fainzilber's research is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; the European Research Council; and the Laraine and Alan A. Fischer Laboratory for Biological Mass Spectometry. Prof. Fainzilber is the incumbent of the Chaya Professorial Chair in Molecular Neuroscience.