Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
All living things – from single-celled algae to our own bodies – are vulnerable to attack by viruses. Dr. Assaf Vardi and his team recently revealed a new mechanism by which viruses infect a marine alga. The results of this research, which appeared in Proceedings of the National Academy of Sciences (PNAS), may help researchers understand cycles of marine algal bloom. The detailed analyses of this unique pathway will also provide a new understanding of the evolutionary “arms race” between viruses and their hosts.
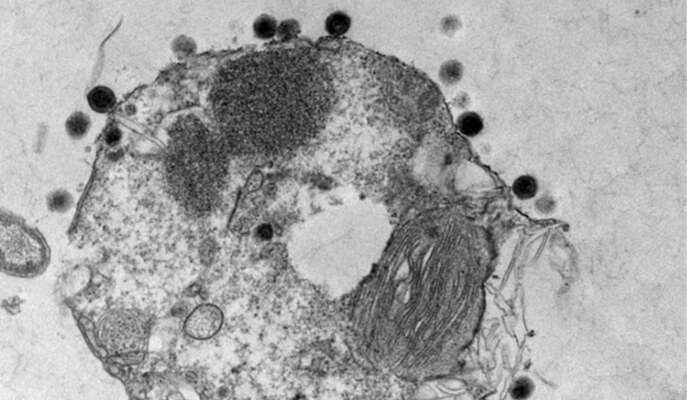
The alga Emiliania huxleyi is one of the more common algae in the ocean. It is responsible for some of the largest blooms – so large they can be tracked by satellite, and they make a significant contribution to the global carbon turnover. The blooms spread for kilometers and then disappear as rapidly as they grew; previous research by Vardi and his team had shown that viruses can spread and kill off an entire bloom within a week or two. Like all viruses, these insert themselves into host cells and hijack the cells’ materials to build copies of themselves. But those that wipe out the blooms are also specialists – uniquely adapted to infecting one particular species of alga.
Researchers, led by Dr. Carmit Ziv of Vardi’s lab in the Weizmann Institute of Science’s Plant and Environmental Science Department, in collaboration with Prof. Asaph Aharoni and Dr. Sergey Malitsky from the Weizmann Institute and Dr. Thorsten Hornemann and Dr. Alaa Othman from the University of Zurich, Switzerland, investigated the genetic sequence of the algal virus. They found that it contained a cluster of genes – encoding a metabolic pathway – that was nearly identical to one in the alga, and they aimed to understand its function during viral infection. This pathway encodes a set of enzymes responsible for the synthesis of a type of fatty molecule, or lipid, called a sphingolipid, that is found in nearly all cells from algal to human. These lipids have several functions in the cell, playing structural roles in the cell membrane as well as signaling functions that help control the cell’s fate. Viruses use these same lipids to build their membranes.

The scientists traced the production of the viral version of these lipids, finding that it is churned out in great quantities as soon as a virus enters an algal cell. As the team continued to investigate, they discovered that these lipids are essential to the process of infection. The virus immediately rewires its host’s lipid-production mechanism, using the viral enzyme to manufacture the one it needs. These lipids then become the bases for assembling new viruses. The viral lipids also act as signals to the host cell – often to initiate the cell suicide program that an alga may opt for in a last-ditch attempt to keep the infection from spreading.
“Our findings provide unique insight into the chemical arms race that continually evolves between virus and host. This virus has evolved to be highly efficient at coopting its host’s lipid production; its survival strategy is thus based on metabolic engineering,” says Vardi. This arms race may indeed be fateful for both cell and virus and thus be responsible in part for the bloom and bust cycles in the ocean. The researchers point out that rises in levels of viral lipids can serve as early indicators of infection in these cycles. This could help quantify the impact of marine viruses on both carbon cycling and the marine food web that relies on these algae.

This is the first time that a metabolic pathway encoded in a viral genome has been found to modulate its host’s metabolic lipid production during viral infection. But because lipids are essential to both cells and viruses, the scientists suggest that other viruses, including those that cause disease in humans, may have evolved similar genetic means for infecting cells by manipulating their hosts’ lipid production.
Dr. Assaf Vardi's research is supported by the Benoziyo Fund for the Advancement of Science; the Angel Faivovich Foundation for Ecological Research; the Rothschild Caesarea Foundation; Dana and Yossie Hollander, Israel; Roberto and Renata Ruhman, Brazil; Selmo Nissenbaum, Brazil; the Brazil-Israel Energy Fund; the Lord Sieff of Brimpton Memorial Fund; the European Research Council; the estate of Samuel and Alwyn J. Weber; and the Germaine Hope Brennan Charitable Foundation. Dr. Vardi is the incumbent of the Edith and Nathan Goldenberg Career Development Chair.