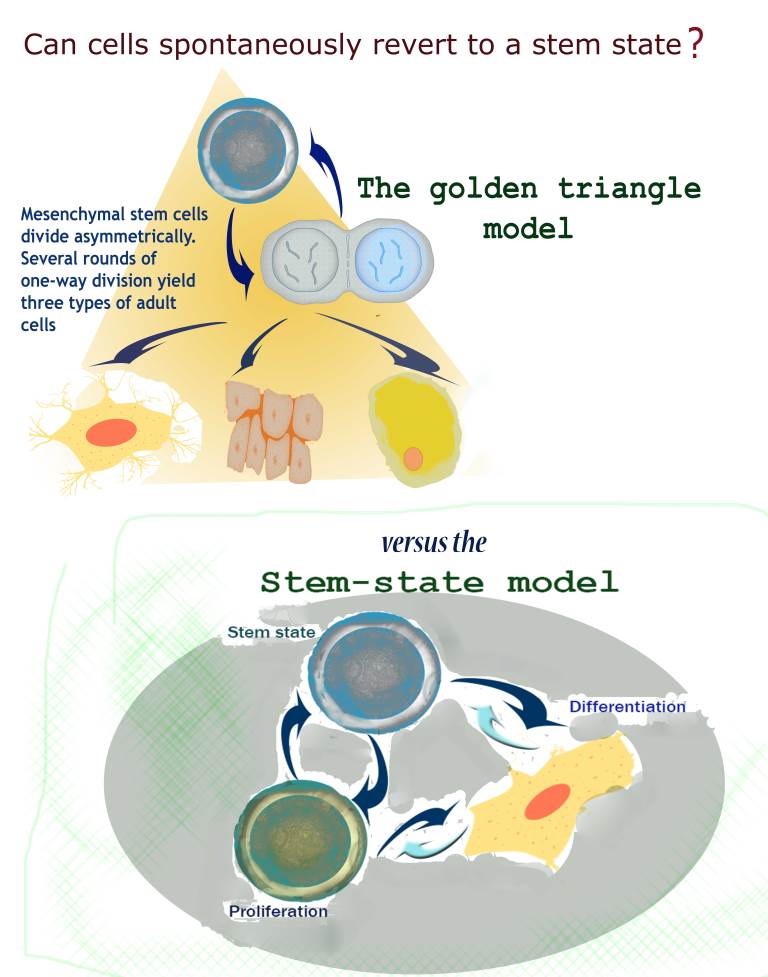
This model – including the "golden triangle" – was mostly set in stone by a scientific paper published in 1999. Mesenchymal stem cells were placed atop the triangle, as they could give rise to any of the three tissue types. Much subsequent research into these cells – and stem cells in general – has relied on this scenario. But Zipori, even then, wondered if these findings truly reflected what happens in the body. For one thing, even though it had not been shown in mammals, fruit fly experiments had found evidence that certain maturing cells in the reproductive system can revert to a stem cell state. And his own experiments with these cells suggested that mesenchymal cells were a bit more unruly than the rigid, one-way model would suggest. He had already noted in the mid-1980s that when the conditions in the cells' environment were slightly changed, some differentiated cells readily lost their mature cell properties and returned to a seemingly undifferentiated state.
By 2004, in
an article he published in
Nature Reviews Genetics, Zipori suggested that researchers begin to think in terms of a "stem state" – a state of the cell – rather than stem cells – an either-or category. Stemness thus implies a fluid condition that a cell might pass into or out of. He also raised the notion that this activity might happen spontaneously in the body. In fact, he wrote, the production of new stem cells could result from maturing cells gaining stem cell potential, rather than from symmetrical stem cell division.
Just two years later, in 2006, the idea of the “stem state” or “stemness” was vindicated when researchers in Japan took mature cells and endowed them with stemness by reverting them back to the very first cell type – embryonic-like stem cells. This was done by artificially imposing specific gene expression in the cells. Nevertheless, few were ready to believe that cells could revert spontaneously in the body from maturity to stemness.
In
the present study, which appeared recently in
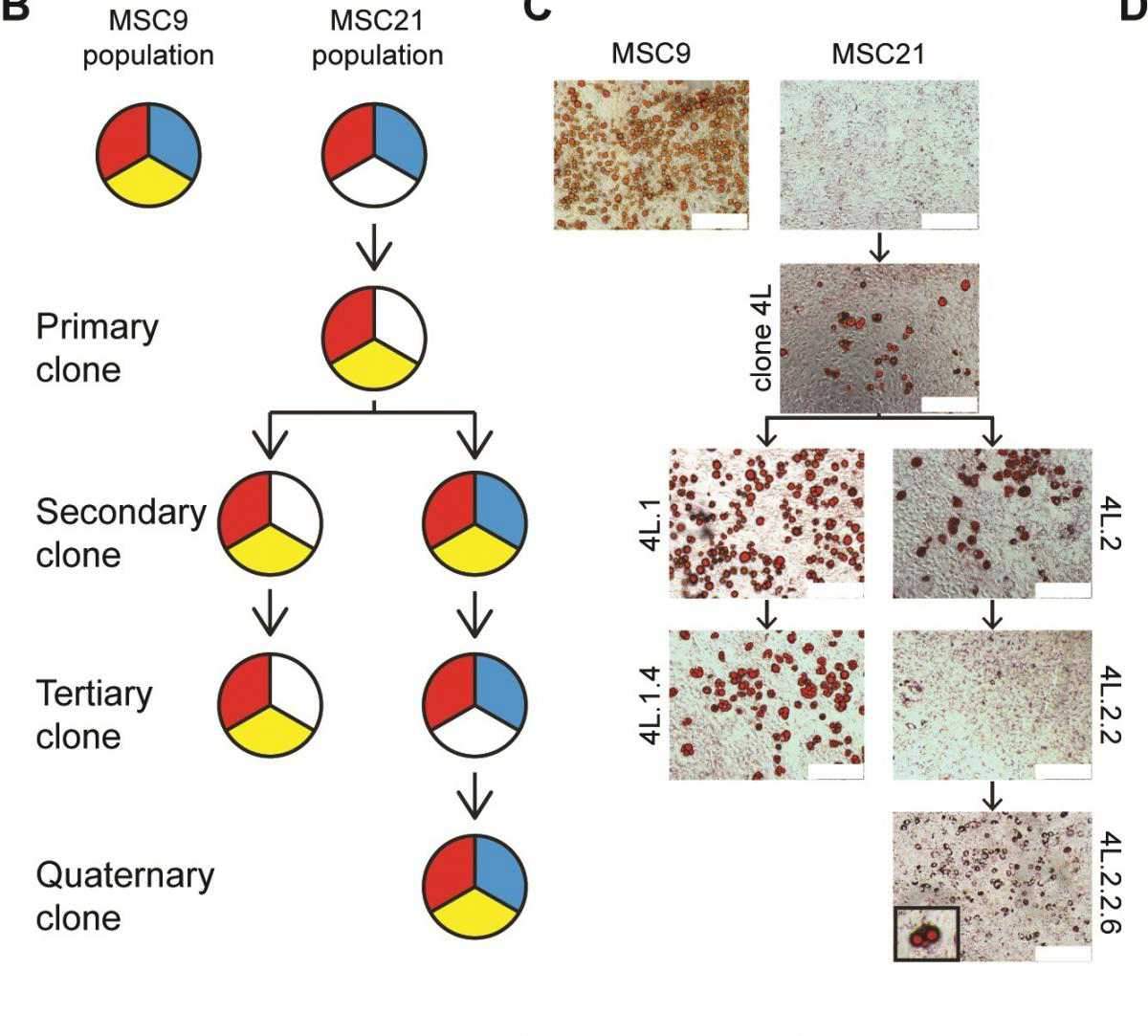
Stem Cell, Zipori and his team, set out to test the true rigidity of the mesenchymal differentiation pathways: Did the commonly accepted paths, with their inflexible chain of events, reflect reality? Following the procedure used in the earlier experiments, the team isolated single mesenchymal cells from mice, cloned them and grew them in lab dishes. But, using modern methods of single cell tracking and analysis, they were able to dig deeper. The study had been initiated five years earlier by Dr. Ofer Shoshani, then a Ph.D. student, who was later joined by additional members of the team, including Dr. Orly Ravid, Hassan Massalha, Alla Aharonov and Yossi Ovadya, and Drs. Meirav Pevsner-Fischer and Dena Leshkowitz of the Institute’s Bioinformatics Unit.
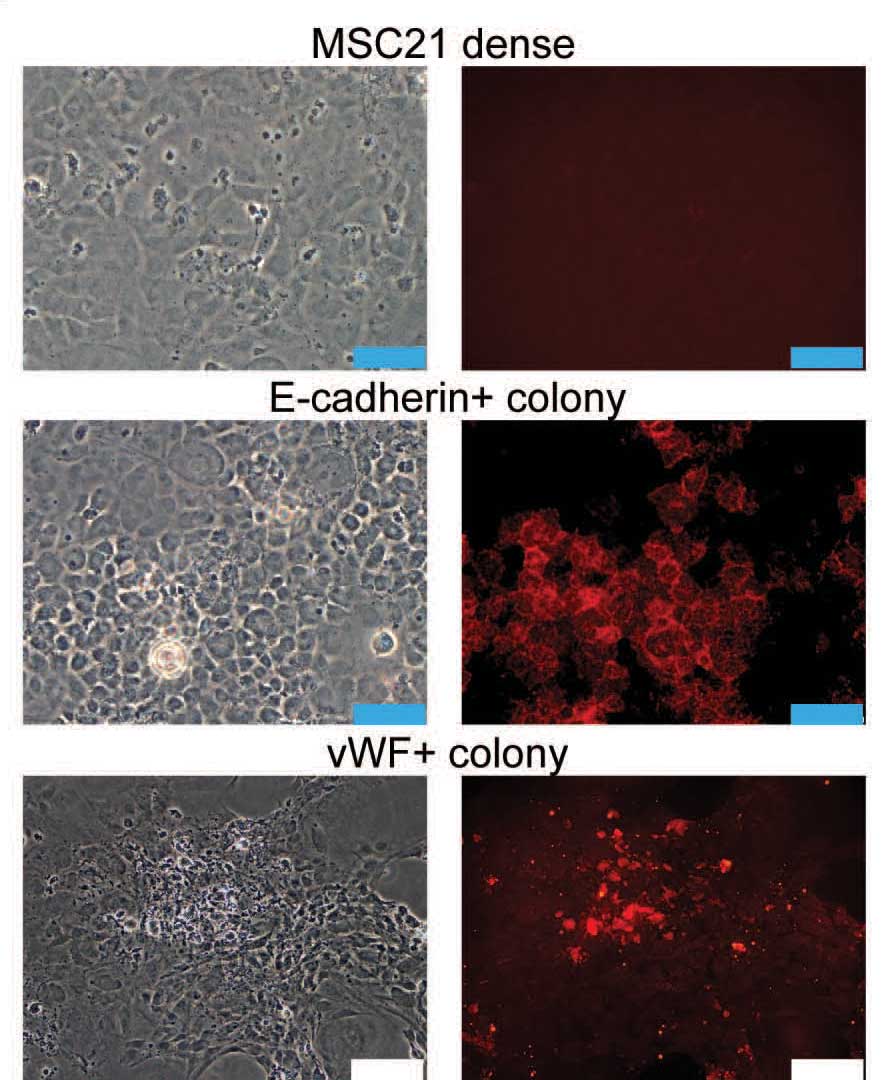
The researchers managed to capture mesenchymal cells in the intermediate stages of differentiation. If the original model was correct, each generation should show a progressive loss of differentiation potential. But what they found was very different: Some cells, indeed, lost a particular sort of differentiation potential – say, the ability to turn into cartilage; but others of their siblings and cousins at each stage regained it. Rather than a linear, one-way street, they now had a more jumbled picture in which cells could go back and forth from one state to another. Although the team was looking at the potential to turn into one of the golden triangle tissues – fat, bone or cartilage – they also saw signs that the mesenchymal cells could give rise to other kinds, for example, endothelial-like or epithelial-like cells.
According to Zipori, these mesenchymal cells may have easily acquired and discarded various states of stemness because they were under stress – in this case the stress of the cell being removed from the surroundings in which it normally functions and placed in a lab dish. This fits in with another observation the team had made: The cells tended to revert much less often when they were grown in low oxygen conditions. Within the body, low oxygen is the default situation for stem cells; high oxygen, in the case of injury, for example, signals stress. To Zipori, these findings hint that the body’s mesenchymal cells may jump back to higher-potential states in such situations as tissue damage, in which many types of new cells may be quickly needed.
Zipori: "Our findings imply that the single-cell cloning used in the past to define mesenchymal stemness may not have discovered true mesenchymal stem cells that give rise to all the differentiated types. Rather, they most likely observed intermediate mesenchymal cells that, because they were removed from their normal environment and thus grown in stressful conditions, had simply jumped back to a higher differentiation potential. Much research has relied on this model over the past 15 years; but our findings suggest that we need to rethink even our most basic assumptions about the ways that cells are renewed in our bodies."
Prof. Dov Zipori’s research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research, which he heads; the J & R Center for Scientific Research; the Leona M. and Harry B. Helmsley Charitable Trust; David and Molly Bloom, Canada; and Roberto and Renata Ruhman, Brazil. Prof. Zipori is the incumbent of the Joe and Celia Weinstein Professorial Chair.