Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
When teams huddle before a game, players are assigned their roles on the field or court. A recent study at the Weizmann Institute of Science and University College London has now revealed what happens when certain immune cells huddle before going out into the body to direct an attack against a pathogen. So-called helper T cells cluster together to decide on the role each will play: Some will manage the short-term concerted attack on pathogens, others the long-term resistance to future infection. The findings of this research, which were recently published in Science, may point the way to better strategies for vaccination, including possibly against diseases for which there is currently no effective vaccine.
In the currently accepted scenario, the individual helper T cells in the lymph nodes get word of an infective agent, divide into new daughter cells, and then go out to direct an attack on the invading virus or bacterium. Though these cells had recently been observed forming clusters within lymph nodes, it had not been clear whether there was any significance to this activity. The new study reveals that huddling is crucial: The helper T cells confer with one another, counting how many cells are in their close environment at any one time and deciding, together, how to proceed with the process of differentiation, in which the daughter cells take on the attributes they need to perform their assigned roles.
Prof. Nir Friedman of the Weizmann Institute’s Immunology Department explains that helper T cells are those that call up and organize the various other cells in the immune response squad, including the B cells that make antibodies, the macrophages that “eat” bacteria, and even other T cells that poison infected and malignant cells. But first the helper T cells must themselves become activated, a process that takes place in the lymph nodes when one of the immune system “scouts,” presents to a T cell a bit of the pathogen’s protein, known as an antigen. As the activated T cells divide within the lymph nodes, each daughter cell carries the information needed to get to the site of the infection and identify the invader. Most of the daughter cells are so-called effector cells that mount the response and die soon afterward. But T cell differentiation also produces long-lived memory T cells that remain in our bodies for years or even decades, in readiness for renewed bouts with the same pathogen.

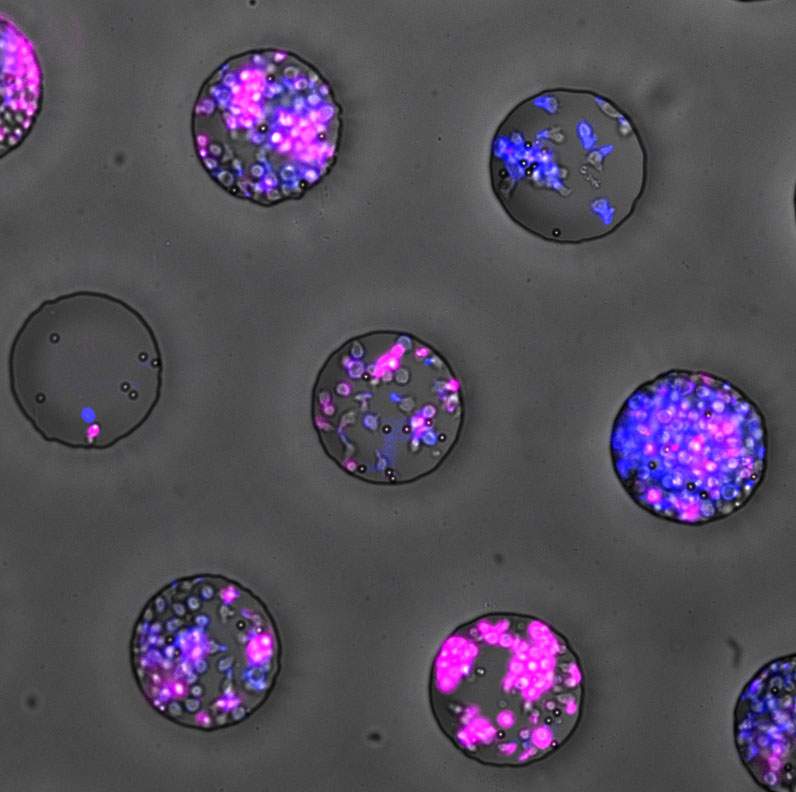
Members of Friedman’s lab, led by research student Dr. Michal Polonsky and in collaboration with the group of Prof. Benjamin Chain of University College London, asked whether the huddling that had previously been observed by Friedman and others played a functional role in the immune response. To experiment with this process and observe it in detail, the scientists turned to a technique that had been previously developed in Friedman’s lab, in which cells are grown in microwells. Plates containing thousands of these wells – indentations less than the thickness of a human hair – are seeded with a few cells each, and the growth and development inside these wells can be followed under a microscope for a period of several days. The method of producing the microwells is based on the technology for making printed electronic circuits; and they are produced in the Institute’s Chemistry Research Support clean labs.
There is really a collective decision-making process taking place. When the numbers are larger, the differentiation is also faster and more efficient
Some of the microwells started out with just one or two helper T cells; others up to ten. After activating the T cells with an antigen, the division, differentiation and clustering were tracked with a microscope. The two types of cells – effector and memory – were color-coded for easy identification.
The researchers noted a distinct difference between wells that started out with only a few helper T cells and those in which the initial conditions were more crowded: The densely populated ones produced many more memory cells. Further data analysis and modeling revealed that the cells can “count.” This is similar to bacteria that are known to engage in “quorum sensing”: Through various environmental cues, they count and then use their tallies to reach decisions that affect the colony – or in the case of T cells, the immune system – as a whole. “This is a more complex scenario than the commonly accepted one,” says Friedman. “There is really a collective decision-making process taking place. When the numbers are larger, the differentiation is also faster and more efficient.”
“The immune system has to allocate its resources,” adds Chain. “Once there are enough T cells to go out and fight the infection, they can then afford to make those memory cells that enable our immune system to fight another day.”
The research team also managed to isolate several of the signaling molecules secreted by the huddled T cells, showing how these helped them “feel” the size of the group and produce more or fewer memory cells as a result.
Today’s vaccinations obtain the memory cells that protect us over time through the standard process of differentiation: They introduce an antigen into our bodies in the form of a killed or weakened pathogen. The T cells in our lymph nodes are then activated, and they divide and differentiate in the same way as if a real threat were imminent. “We might be able to use this knowledge to tip the immune response in vaccinations toward producing more memory cells and less immediate immune response,” says Chain. “The findings might also help in developing new vaccines for diseases that are not prevented today or for people, such as the elderly, in whom the immune response is already weaker, and they might also lead to new directions in cancer vaccination” adds Friedman.”
Prof. Benjamin Chain, a professor of immunology at University College London, has been spending a month each year in Friedman’s lab in the Weizmann Institute of Science since they first met around a decade ago. In that time, the labs have exchanged both students and ideas. Their ongoing collaboration has been facilitated, in part, through the “Making Connections” program, an initiative of Weizmann UK. Chain had already noted clustering among immune cells called dendritic cells as far back as the 1980s, but science labs did not have the resources at that point to uncover exactly what those cells were doing. “It is only with today’s advanced techniques, and both labs working together, that we could really investigate them on the level of single cells or small groups, and over time. This includes the development of the advanced dedicated computational tools we have available to us for analyzing the huge datasets generated by the videos of thousands of microwells over several days,” he says.
Prof. Nir Friedman's research is supported by the David and Fela Shapell Family Institute for Preclinical Studies; the Florence Blau Charitable Trust; the Bernard and Audrey Jaffe Foundation; and the Park Avenue Charitable Fund. Prof. Friedman is the incumbent of the Eugene and Marcia Applebaum Professorial Chair.