Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Microglia – hairy cells arrayed in dense formation deep within our brain tissue – seemed, in the standard still images, to sit so quietly in place that for years they were, alongside other non-neuron cells, thought to be mere “glue,” or glia. But researchers now know that microglia, which are in fact immune cells, are truly always very busy scanning their neighborhood, shaping synapses and clearing up dead cells. A new study at the Weizmann Institute of Science provides fascinating insights into the way in which microglia regain their cool after briefly firing up in response to a challenge.
“Since most neurological conditions, from ALS to schizophrenia and Alzheimer’s, involve the need to keep the microglia quiescent, we think that understanding the shutting-down side of a response is as critical as the start,” says the lead author of the study Prof. Steffen Jung of the Immunology Department.

When microglia mount a full-on immune response, it is a hot, rapid flare, involving changes to the expression of thousands of genes. What then shuts down this response, allowing these cells to return to their normal, resting state? Because microglia belong to the type of cells called macrophages – the “big eaters” that devour unwanted substances or cells in various body tissues – Jung and his team thought that their shutdown might involve the anti-inflammatory substance interleukin 10 (IL-10), which they knew from their earlier studies plays a role in regulating gut macrophages. Despite the fact that microglia are extraordinarily long lived and quiescent, while gut macrophages are short lived and twitchy, the team’s earlier experiments had found that these two cell types use similar tool sets to function.
“We thought of these cells as atoms, which tend to return to their ‘ground state’ following excitation,” says Jung. “But atoms do it on their own, whereas we have found that microglia need external help to restore quiescence, once they are ‘excited.’”
When microglia mount a full-on immune response, it is a hot, rapid flare
The research team, including Anat Shemer, Gal Ronit Frumer and other members of Jung’s lab, working with the research group of Dr. Kiavash Movahedi in the University of Brussels, created mice in which they could control the IL-10 signals just in the brain. When they ablated the IL-10 receptor on the microglia, the mice appeared to be normal – that is, until they were challenged with LPS, a bacterial cell wall component that is known to induce an extreme immune reaction known as a cytokine storm and to incite the microglia. Now these microglia, unable to sense IL-10, remained in their “excited” state, and the mice developed signs of neurological disease. This established the fact that IL-10 plays a specific role in the brain, bringing active microglia back to their normal, quiet states that are clearly critical for health.
Next, the research team asked where the IL-10 comes from. Their experiments showed that this cytokine was not produced by the microglia themselves, but rather by other immune cells. The excited microglia, however, produced a different substance, TNF, which causes inflammation. Indeed, when the microglia could not sense IL-10, owing to the missing receptor, the cells became hyperactive, overproduced TNF and as a result, the animals displayed signs of pathology. In contrast, mice with microglia lacking both TNF and IL-10 receptors showed no signs of inflammation or disease. In other words, the researchers revealed the following sequence of events: The excitation of microglia causes them to produce TNF, which is deleterious unless production is shut off by IL-10, which brings the microglia back to the ground state. As always, many questions remain: What, for instance, happens to this circuit in various neurological disorders? And why do the microglia not make their own shut-off substance, as do other macrophages, including those in the gut?
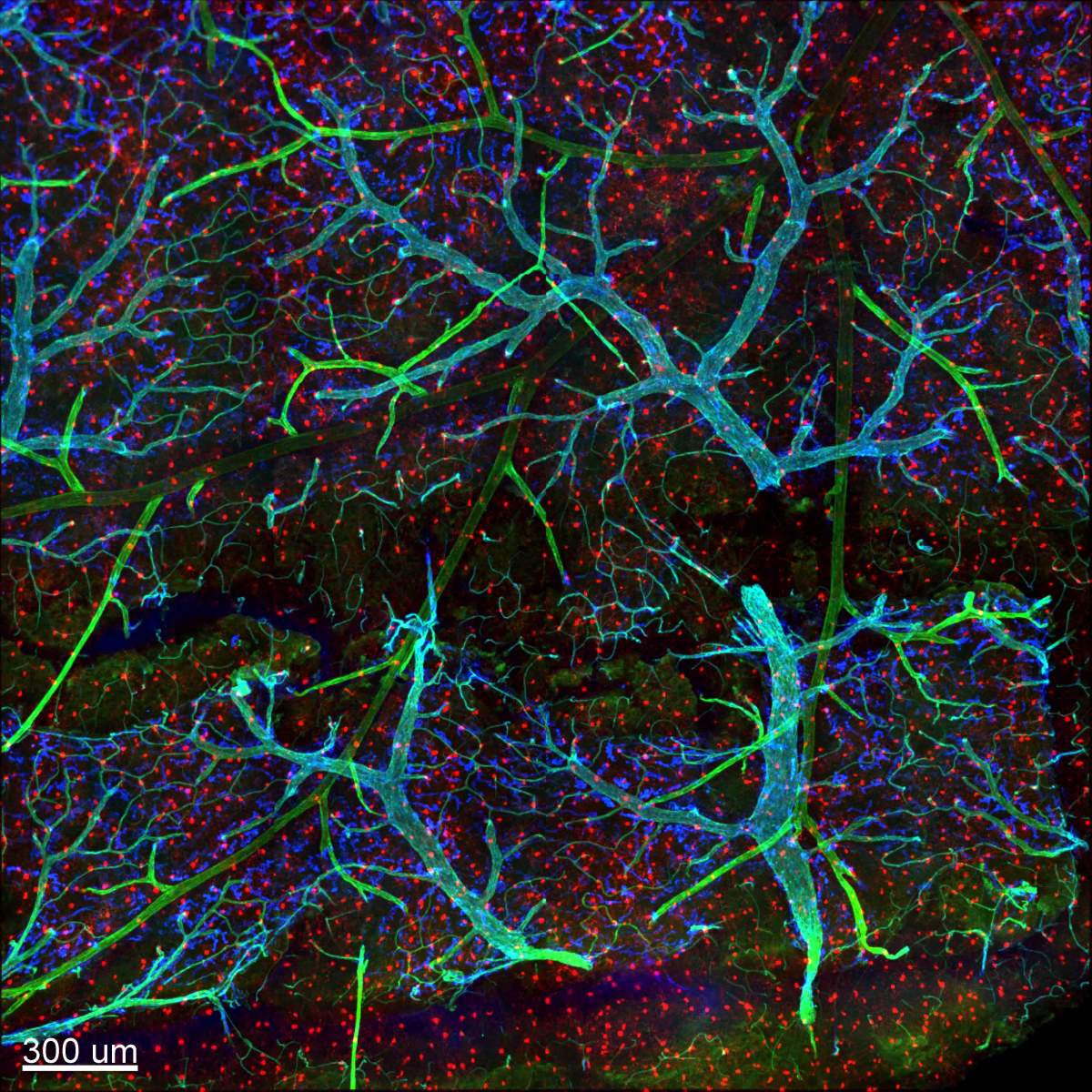
This latter discovery indeed came as a surprise, since other labs had reported IL-10 being produced by microglia when these cells were cultured in standard lab dishes. The finding that they do not do so in brain tissue supports the view that microglia – and, indeed all macrophages – take their very identities from the information they sense in their microenvironment, something that is not provided in lab dishes. Jung suggests that there is a reason for microglia forgoing IL-10 production: It enables the cells to give their all to the response and ensure the delay in their return to the ground state.
For the IL-10 study, Jung and his team had created lab mice in which the IL-10 receptors were absent from brain macrophages, but not macrophages in the gut, where the lack of the receptor would produce severe colitis. But the genetic manipulation in this study not only affected microglia but also other macrophages in the brain, including the perivascular cells that line the blood vessels. To address this issue, Jung-Seok Kim in Jung’s lab led a heroic effort to develop an approach for manipulating genes in just one type of brain macrophage, either microglia or perivascular macrophages..
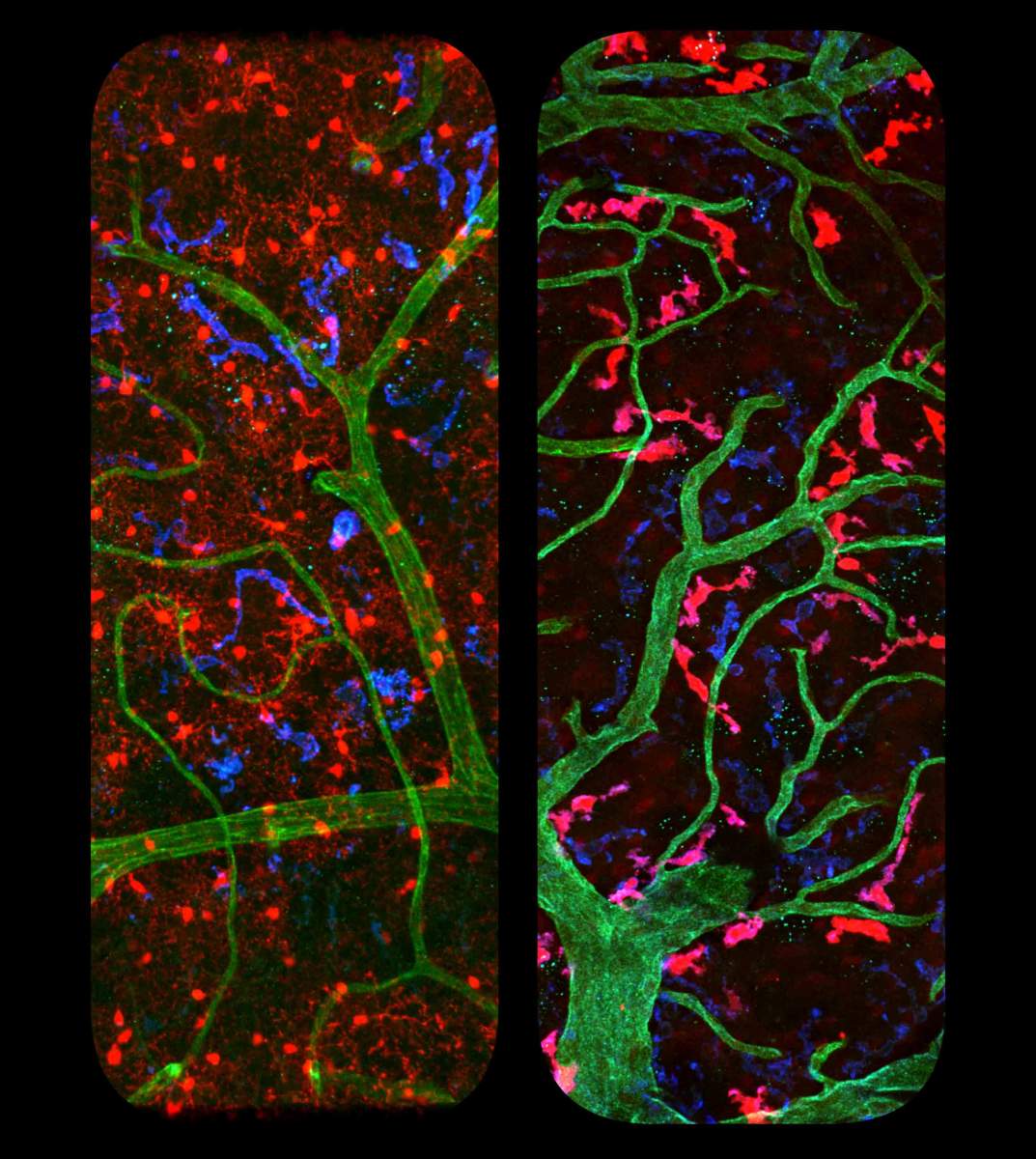
The classical method for gene manipulation in the mouse involves the use of Cre recombinase, a protein that, as its name suggests, can switch around or rearrange genes. To activate the recombinases, the researchers hook them up to promoters within the genome that function as “on” switches and allow researchers to control the timing, location and level of the gene modification. However, as is the case with the brain macrophages, promoter activities are rarely cell-type specific. Jung and his team therefore decided to split the recombinase into two halves, each part unable to act on its own. This allowed them to place the parts under the control of distinct switches that discriminated between microglia and perivascular cells in the brain. When both promoters were activated, the recombinase fragments came together, giving the researchers a tool to make alterations in one type of immune brain cell alone.
In their first report of the model, a collaboration with Dr. Pablo Blinder at Tel Aviv University and Dr. Kiavash Movahedi at the University of Brussels, the research team found that these two brain cell types not only live in different surroundings but also have distinct shapes and take on quite different functions. With this method, researchers can now ask questions about, among other topics, how the perivascular cells, which are first responders to messages carried in the bloodstream, and the quiet, resting microglia communicate and coordinate their activities with one another. Jung and his team believe other research teams will adopt this method so as to pinpoint the exact role of different immune cells in health and disease and, in the future, design treatments for cell-type-specific imbalances.
Prof. Steffen Jung is Head of the Kleinman Cancer Cell Sorting Facility; and his research is supported by the Morris Kahn Institute for Human Immunology; the Maurice and Vivienne Wohl Biology Endowment; the Roland N. Karlen Foundation; and the Blythe Brenden-Mann Foundation. Prof. Jung is the incumbent of the Henry H. Drake Professorial Chair of Immunology.