Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Resplendent multicolor cone-snail shells harbor a deadly predator that kills fish, worms and other prey with precision and speed. In a study published in the Proceedings of the National Academy of Sciences, (PNAS) USA, researchers at the Weizmann Institute of Science have revealed how cone-snail venom does its lethal job – and discovered that it blocks potassium channels in the cell via a previously unknown mechanism. The discovery may help explain some of the side effects of existing potassium channel-blocking drugs, and it may lead to the development of new drugs to induce this blockage in innovative ways.
“Certain components of the cone-snail venom kill insects but not mammals,” says Dr. Izhar Karbat, a staff scientist in the laboratory of Prof. Eitan Reuveny of the Biomolecular Sciences Department. “The original idea of the study was to try to use these components for developing natural pesticides that would kill pests without harming humans.”

Toxins found in the venom of many venomous organisms paralyze victims by plugging the opening, or pore, of potassium channels – cellular structures that regulate the transmission of neuronal signals, electric stimulation of the muscles and numerous other processes by letting potassium ions flow into and out of the cell. Some potassium channel-blocking drugs – including those that treat blood pressure and abnormal heart rate – work via the same mechanism, often known as “a cork in the bottle.” So when Karbat and co-workers set out to study the venom of cone snails, the first step was to check whether it, too, works like a plug.
But experiments in the lab showed that the venom doesn’t work by the cork-in-the-bottle principle. How, then, does it paralyze and kill its victims?
Karbat and other Weizmann team members, working with researchers from Tel Aviv University and the University of Debrecen, Hungary, produced crystals of key toxins from the cone-snail venom, and solved the three-dimensional structure formed by these toxins. Then they used electrophysiological recordings that assessed the function of the channel and created mutations that provided information about the contribution of various regions in the toxins and the channel proteins to their mutual interaction. The results suggested that the toxins interact with elements on the periphery of the channel rather than with its ion permeation pore, which is formed from four symmetrical channel subunits.
Next the scientists performed computer simulations using advanced versions of a technique called molecular dynamics, which enabled them to examine the interactions between the venom and the channel’s proteins at the level of individual atoms. “One of the advantages of this technique is that it exposes the locations not only of protein elements, but also of individual water molecules and ions,” Karbat explains.
Insights drawn from the toxin action could point to ways of avoiding these unwanted side effects
The simulations revealed that interactions between amino acids of the toxins and those of the channel proteins altered hydrogen bonds within the channel in a manner that increased the flow of water in protein cavities around the channel pore. The increased flow, in turn, ultimately caused the entire channel pore to collapse, preventing it from conveying further potassium ions from one side to the other. In other words, the channel stopped functioning and the flow of potassium ions was blocked – not by a cork-in-the-bottle, but by a previously unknown mechanism that took effect on the periphery of the channel’s pore.
This discovery sheds new light on the way potassium channels are regulated in nature. For example, it was known that the channels may shut down as a result of a prolonged opening of the channel and increased ion flow, leading to its collapse – a process called slow inactivation – but the underlying molecular mechanism of this process was unknown. The new findings suggest that slow inactivation is probably caused by an increased water movement and the resultant structural changes on the periphery of the channel pore.
Moreover, the discovery provides valuable insights for drug design. It might, for example, be possible to test whether some of the side effects caused by potassium channel-blocking drugs are due to structural changes that resemble those induced by the cone-snail toxins. Insights drawn from the toxin action could then point to ways of avoiding these unwanted side effects. The pore of the potassium channel has been conserved throughout evolution – that is, it has the same composition and structure in different organisms and in different types of cells – which means that a drug that works by blocking the pore is likely to affect all these types of cells in such tissues as the brain, muscle and kidney. In contrast, the protein parts of the channel periphery, away from the pore, vary greatly between different types of cells and tissues and thus may become a possible selective target for drugs.
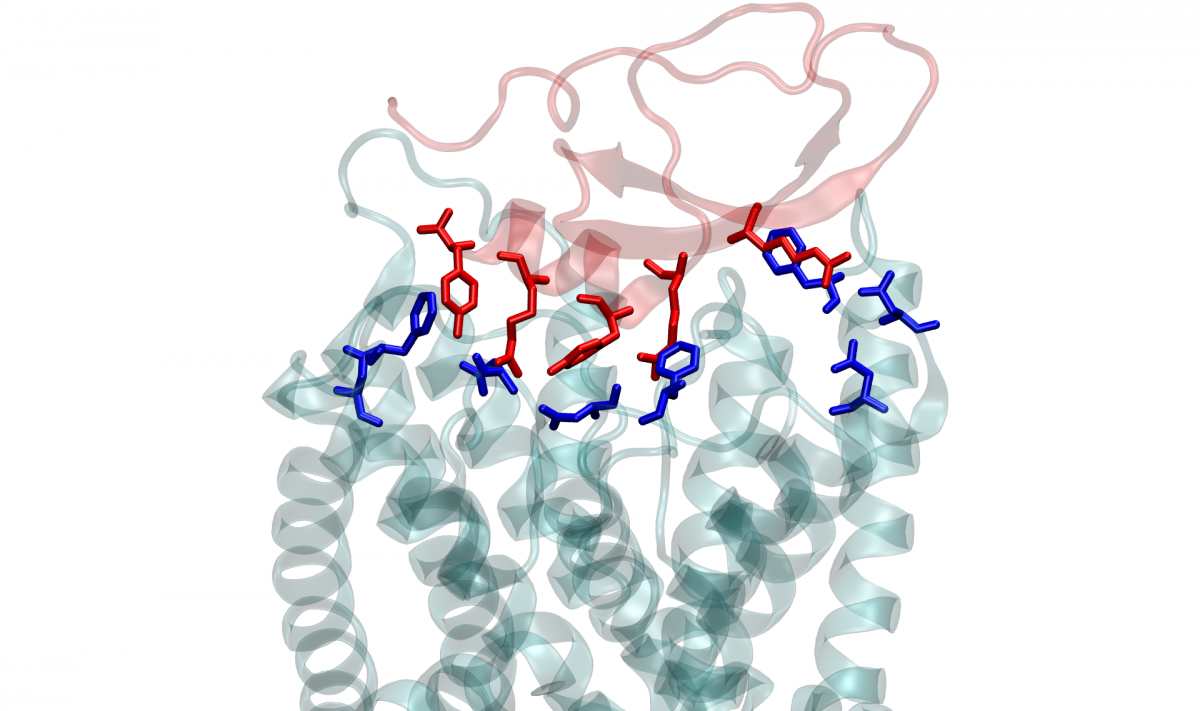
“If small-molecule drugs are designed to alter the periphery of the channel, causing the pore to collapse, it may be possible to target subsets of channels that are selectively expressed in specific cells,” says Reuveny. “And this, in turn, may reduce the side effects of the drugs.”
Study authors included Shachar Fine and Dr. Dalia Gordon of Weizmann’s Biomolecular Sciences Department; Shelly Hamer-Rogotner and Dr. Orly Dym of Weizmann’s Dana and Yossie Hollander Center for Structural Proteomics; Hagit Altman-Gueta, Dr. Felix Frolow and Dr. Michael Gurevitz of Tel Aviv University; and Tibor Szanto and Dr. Gyorgy Panyi of the University of Debrecen.
Prof. Eitan Reuveny's research is supported by the Schwartz/Reisman Collaborative Science Program; and the Nella and Leon Benoziyo Center for Neurological Diseases.