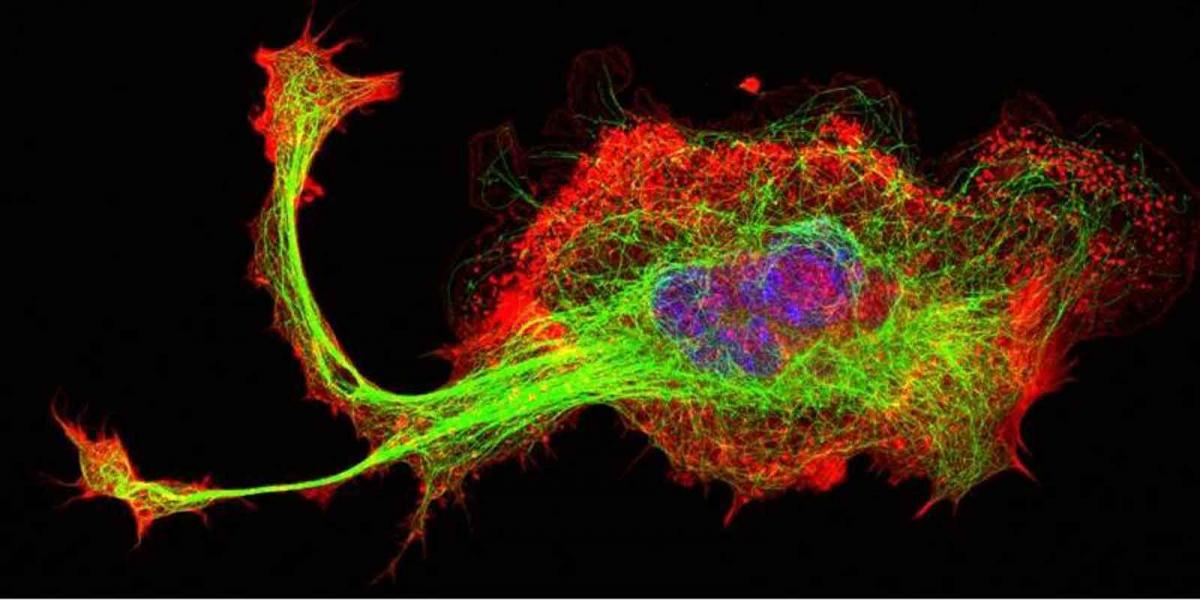
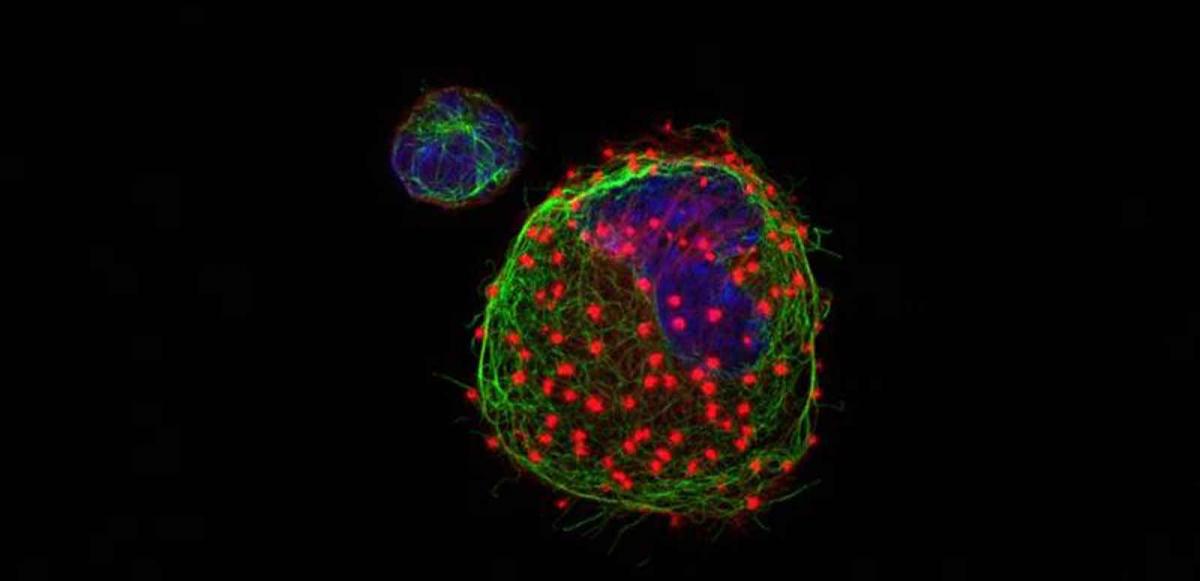
Using a large array of techniques in their labs, the researchers were able to reveal the precise activities of miR-142.
Their findings, which were recently published in
eLife, show that miR-142 is, indeed, a master key that turns on and off a number of different cellular processes; these are crucial to actin production and regulation. To put it another way, microRNA-142 is a “hub” in the cellular network of pathways that keeps the cell growing, dividing, developing and functioning.
According to Hornstein, the impact of microRNA-142 and its mechanism may even go all the way back to the first blood cells in the embryo. In addition, miR-142 malfunctions are likely to show up in certain clotting disorders; but the findings hint that the same miRNA gene may be involved in any number of other blood diseases. Hornstein: “This model for blood cell development is very informative and fruitful. Together with Jung we have already characterized four different cell types in which this miRNA is influential, which is very exciting.”
The implications are clear for microRNA research, says Hornstein, helping cast microRNA in a new light: they can no longer be seen as mere helper molecules that “fine-tune” the cellular pathways; they are also key players with the power to direct the development of the cell.