Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
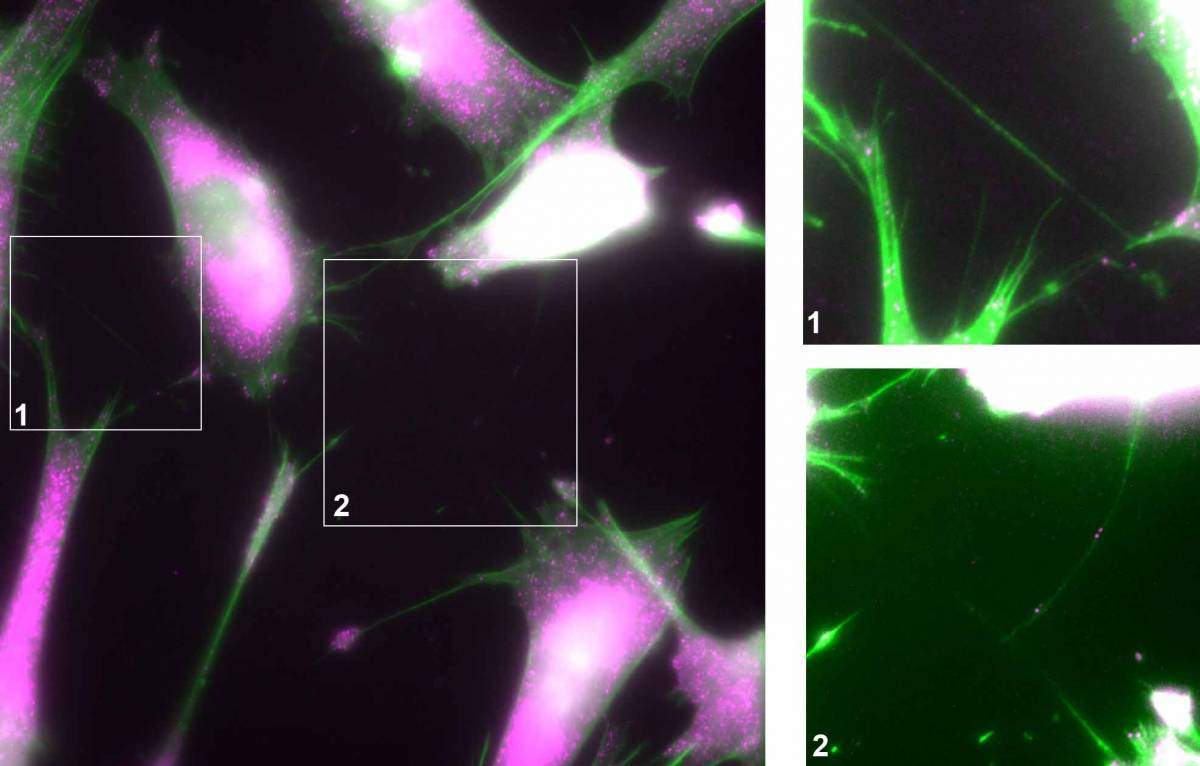
Cells have numerous means of communication, but one discovered in recent years had researchers surprised: Cells can extend small tubular structures – a sort of nanotube – between their membranes to enable the direct transfer of material from one to the other. A network of cells, or “internet of cells” as it was dubbed in the journal Nature, can connect up by nanotubes; but like certain kinds of online interactions, this has both potential benefits and risks. Weizmann Institute of Science research recently added to the mystery by showing that the material passing through these nanotubes includes genetic instructions that can directly alter the internal functions of the recipient cell. These findings, say the researchers, call into question the very idea of the cell as a walled-off, autonomous unit.
Prof. Jeffrey Gerst of the Institute’s Molecular Genetics Department says the nanotubes function something like the jet bridges, or “sleeves,” that we walk down to board a plane directly from the gate. For airports, this makes boarding more efficient than the older method of bussing passengers to the plane, but it does mean that the planes must always be next to the gates. Indeed, cells employ “buses” – small, enclosed vesicles that transport various molecules from membrane to membrane. Vesicles even do, on occasion, transport genetic material in the form of small RNAs; but this RNA is generally not of the kind known as messenger RNA, which carries instructions for protein manufacture from the genes to the protein-making machinery.
The new study, led by postdoctoral fellow Dr. Gal Haimovich in Gerst’s lab, shows for the first time that cells can also transfer whole messenger RNA molecules from cell to cell – not in vesicles, but directly through the nanotubes. In other words, an order for protein manufacture can be issued in one cell and carried out in the machinery of the next. The findings of this study were published in the Proceedings of the National Academy of Sciences (PNAS).
An order for protein manufacture can be issued in one cell and carried out in the machinery of the next
Haimovich began his research in New York, in the lab of Prof. Robert Singer of the Albert Einstein Medical School. His original motivation was simply to understand the mechanics of cell-to-cell communication. “Rather than the usual biochemical or RNA sequencing methods, we developed one that would enable us to see this communication with our own eyes,” he says. The researchers grew cultures of two groups of cells, one of them genetically engineered so that specific messenger RNAs in them could be visualized using fluorescent probes or reporters. The group was stunned by the results: “From the very first cultures, we could see the transfer of entire molecules, and the whole process was quite rapid – it took less than half an hour,” says Haimovich. “As an added plus, we were able to count the molecules, a feat that is not possible with other methods.” As the researchers continued to experiment, they found that messenger RNA was transferred between different types of mouse cells, and even from mouse to human cells.
What was not yet clear was how the RNA molecules moved so efficiently from cell to cell. RNA transfer did not occur in experiments in which the cells were removed to opposite sides of the culture dishes, so vesicle “buses” were ruled out as the means of transportation. Next, Haimovich turned to the literature on cellular communication, and that is when he came upon papers describing the discovery of membrane nanotubes. He returned to his results, and sure enough, he discovered thin nanotubes that appeared to connect the cells. When he prevented the formation of the nanotubes, the messenger RNA stayed confined to its original cells, showing that the nanotubes were, indeed, the conduit for passing genetic information.
The idea of functional messenger RNA being shared through an internet-like nanotube network is already raising controversy in the scientific community. Some, says Gerst, have suggested that the phenomenon occurs only in the lab dish; however, nanotubes have been observed in living organisms. Others have embraced the finding, even suggesting that it challenges our very idea of a cell’s individual “identity” and of its function within the “community” of cells in an organism.
Whether messenger RNA is transferred in this way between the cells in our own bodies, how much and in what circumstances – all of these are questions for future research. Gerst: “It could, for example, play an important role in embryonic development, when the process of differentiation needs to be tightly coordinated between cell layers. And we know that cancer cells communicate directly with the healthy cells in their environment, so this mechanism might allow them to quickly subvert their neighbors to their unhealthy schemes.” Gerst and Haimovich add that in the future, treatments might be developed based on the use of membrane nanotubes to deliver corrective genetic information to diseased cells.
Prof. Jeffrey Gerst's research is supported by the Moross Integrated Cancer Center; the Miles and Kelly Nadal and Family Laboratory for Research in Molecular Genetics; the Joel and Mady Dukler Fund for Cancer Research; and the Jean-Jacques Brunschwig Fund for Molecular Genetics of Cancer. Prof. Gerst is the incumbent of the Besen-Brender Professorial Chair of Microbiology and Parasitology.