Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Vascular-targeted photodynamic therapy (VTP) with TOOKAD®, a therapeutic platform for solid cancers developed at the Weizmann Institute of Science, has already gained approval in Europe, Israel and Mexico for the treatment of early-stage prostate cancer and is under examination by the US Food and Drug Administration.
TOOKAD VTP works by triggering the rapid destruction of the tumor’s blood supply, temporarily turning the blood into a generator of toxic oxygen molecules called radicals and shortly afterwards causing blood vessels to collapse. But until now there was no reliable way of learning how exactly Tookad VTP accomplishes this feat. In a new study reported in Nature Biomedical Engineering, researchers in the United States together with the laboratory of Prof. Avigdor Scherz at the Weizmann Institute of Science, applied an innovative imaging technique to follow with great precision and in real time the changes in the tumor’s vasculature that eventually result in the selective destruction of a tumor, without hitting surrounding tissues.
Tumors with a poorer vessel network were less responsive to the treatment than those with a richer vessel network
Tookad – invented by Scherz, of the Plant and Environmental Sciences Department, and the late Prof. Yoram Salomon of Biological Regulation Department – is derived from the chlorophyll found in light-harvesting bacteria. The drug is given intravenously and stays in the patients’ blood vessels for a short while before being cleared from the body. It remains inactive until illuminated by laser light, which causes it to react with the blood’s oxygen, converting it into destructive oxygen radicals that initiate the generation of other toxic but short-lived materials; these are confined to the tumor. These locally-formed toxins attack the tumor’s blood vessels and self-propagate into the tumor’s core, killing the rest of the tissue and depriving the cancerous growth of nutrients and oxygen. Monitoring what happens to the tumor after the drug is illuminated, and in particular, how the self-propagation causes damage, was until recently impossible because available technologies lacked the temporal and spatial resolution for imaging within a living body.
Lead study author Dr. Jan Grimm of the Memorial Sloan Kettering Cancer Center, New York City – where Tookad is now being clinically explored – undertook a study of the drug’s mechanism of action using a new imaging approach: raster-scanning optoacoustic mesoscopy (RSOM). This method involves exciting tissue with pulsed laser light, which causes light-absorbing molecules within – in this case, hemoglobin – to expand. The RSOM then monitors the ultrasound waves created by this expansion. The goal was to obtain a high-resolution picture of blood vessels in the tumor and the surrounding tissue before, during and after treating mice with the drug. As Grimm wrote in a blog post, he got the idea for the study after discussing RSOM with Prof. Vasilis Ntziachristos of the Technical University of Munich, who spent a sabbatical in his lab. He then joined forces with Ntziachristos and Scherz’s lab.
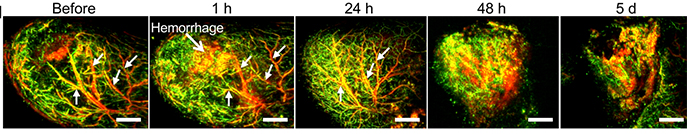
“RSOM allowed the generation of outstanding images of tissue response and microvascular changes after therapy,” Grimm wrote in the blog. The images showed that soon after Tookad was administered to mice, the large blood vessels feeding their tumors became blocked, whereas smaller vessels within the tumors gradually started breaking up. The resulting bleeding introduced pulses of free oxygen molecules into the tumor’s microenvironment, causing further destruction and killing – as typically happens when an oxygen-deprived area is suddenly flooded with blood – while the tumor’s entire network of vessels ultimately collapsed. These changes culminated in the death of tumor tissue.
Tumors with a poorer vessel network were less responsive to the treatment than those with a richer vessel network, underscoring the significance of turning the tumor vasculature into a temporary “death generator.” This revelation calls for new research aimed at amplifying the impact of TOOKAD VTP on tumors with a poor vascular supply.
The study’s unprecedented level of detail may help to optimize the treatment. In particular, using RSOM to monitor the state of the tumor’s blood vessels in human patients may make it possible to predict the treatment’s effectiveness, adjust it in a way that will ensure the tumor’s eradication, on the one hand, and avoid overtreatment, on the other, so as to prevent any collateral damage in the surrounding normal tissue.

Study participants included Dr. Katja Haedicke, who was initially trained in Scherz’s lab at the Weizmann Institute, Dr. Magdalena Skubal, Dr. Sheryl Roberts, Camilla Longo-Machado, Karan Nagar, Hsiao-Ting Hsu, Dr. Kwanghee Kim, Dr. Thomas Reiner and Dr. Jonathan Coleman of Memorial Sloan Kettering Cancer Center; Dr. Lilach Agemy of Weizmann Institute of Science’s Plant and Environmental Sciences Department; and Profs. Murad Omar and Andrei Berezhnoi of the Technical University of Munich.
Prof. Avigdor Scherz's research is supported by the Thompson Family Foundation; Sharon Zuckerman; Lord and Lady Sharp of Grimsdyke; the Samuil and Petr Polsky Prostate Cancer Research Fund; and the Lotte S. and Felix Bilgrey Memorial Fund.