Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Steba Biotech, a privately-owned company, in collaboration with scientists at the Weizmann Institute of Science, has conduct extensive research and development on TOOKAD®, and has received marketing authorization to make TOOKAD® available in 31 European countries. Following this regulatory approval, the first patients are currently being treated in top Israeli, German, English and Italian medical centers.
Moreover, a training center for urologists who want to use the method has been established at the University Hospital of Angers, France, by Prof. Azzouzi.
Every year in the European Union about 365,000 men are diagnosed with prostate cancer; some 77,000 die of the disease. Up to 35% of patients are diagnosed at the “low risk,” early stages; these patients have a much better prognosis than patients in the later, metastatic stages. But these men face a difficult choice: either undergo invasive surgery or radiation, both of which may result in such severe side effects as erectile dysfunction and urinary incontinence; or else adopt the “active surveillance” approach, monitoring the cancer’s progression and delaying radical treatment. This approach, while more risky and do not avoid cancer progression, can preserve the quality of life for as long as possible.
Vascular Targeted Photodynamic therapy (VTP) with TOOKAD® offers a middle way with minimally invasive treatment. It ablates only the prostate lobe that contains the cancerous tissue while preserving the integrity and functionality of the surrounding healthy tissue. The safety and efficacy of TOOKAD® VTP were demonstrated in a randomized controlled trial of 413 patients (level one evidence), across 10 countries (Azzouzi, et.al Lancet Oncol., Feb, 2017) TOOKAD® reduces the progression of cancer and the need for radical therapy, thus preserving of erectile and urinary function in the overwhelming majority of the treated patients. Following the recent marketing authorization from the European Commission, Steba Biotech, which produces TOOKAD®, is currently making the treatment available to a number of leading European medical centers.
TOOKAD VTP was invented by Profs. Avigdor Scherz and Yoram Salomon of the Plant and Environmental Sciences, and Biological Regulation Departments respectively, at the Weizmann Institute of Science. It was translated to clinical use through an extensive collaboration with Steba-Biotech, which undertook clinical development through the first successful phase III clinical randomized trials for low risk prostate cancer.
How does it work? TOOKAD® is a novel drug derived from the chlorophyll found in light-harvesting aquatic bacteria (bacteriochlorophyll). Upon intravenous administration (IV), the TOOKAD® makes complex with the protein serum albumin in the blood, and the complex circulates for several hours before being eliminated. The drug is inactive unless illuminated with non-thermal light; when activated by a laser light, TOOKAD® reacts with oxygen in the blood, converting it into active molecules called radicals. This initiates a cascade of biological events that results in a permanent blockade of the tumor’s blood vessels that initiate a chain of biological events that kill the tumor cells. Within several hours, the tumor undergoes non-thermal necrosis and eradication. This means the treatment can be highly efficient with limited side effects, for example, the treatment avoids erectile and urinary dysfunction in the vast majority of patients, and there are no long-term issues related to the toxicity of the drug, itself. This was confirmed in a four-year follow-up study, published in The Journal of Urology.
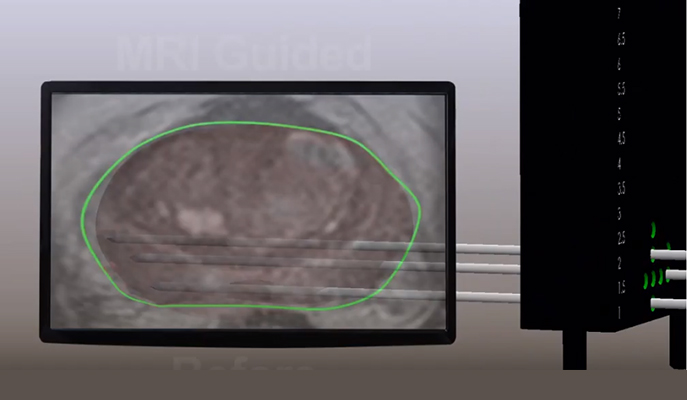
To administer the treatment, thin optic fibers connected to a near-infrared laser are inserted into the cancerous area in the prostate, with the help of magnetic resonance imaging and ultrasound guidance. TOOKAD® is intravenously administrated for 10 minutes, under general anesthesia, after which the laser is turned on for another 22 minutes, to activate the drug locally. In the currently approved focal therapy setting, TOOKAD® VTP can be performed in an outpatient procedure lasting around 90 minutes. Patients are released a few hours later ‒ up to a day ‒ and they can return to normal activities within few days. This new, minimally invasive technology offers a unique alternative to patients diagnosed with early-stage prostate cancer.
In the past few years, Steba has conducted extensive, successful clinical trials of TOOKAD® for prostate cancer in leading hospitals in Europe and in Latin America, as well as in the USA in Memorial Sloan-Kettering, in New York. Hundreds of men who have undergone TOOKAD® treatment are cancer free, or have control of their disease progression ‒ avoiding or delaying the need for more radical approaches and suffering few-to-no side effects of the treatment, itself. TOOKAD® is approved for use in 31 European countries, Israel and Mexico.
Yeda Research and Development Company, the Weizmann Institute’s technology transfer arm, has exclusively licensed the drug to Steba Biotech worldwide. Yeda and Steba Biotech have collaborated on developing the treatment for over 20 years and continue to collaborate today on exploring further cancer applications and getting the treatment to those who need it.
Prof. Avigdor Scherz's research is supported by the Thompson Family Foundation; the Leona M. and Harry B. Helmsley Charitable Trust; Sharon Zuckerman; Lord and Lady Sharp of Grimsdyke; the Samuil and Petr Polsky Prostate Cancer Research Fund; the Lotte S. and Felix Bilgrey Memorial Fund; and the Berdie and Irvin Cohen Weizmann Institute Research Fund.