Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
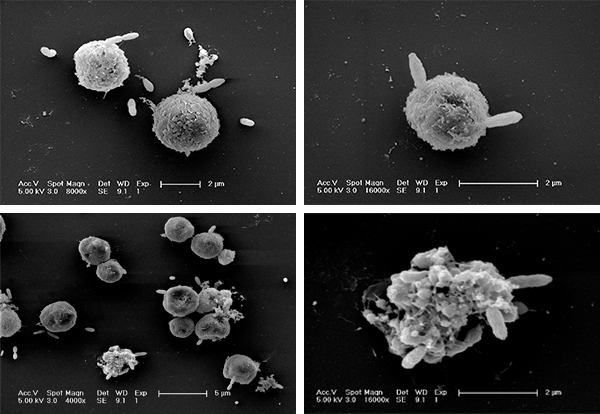
When a giant algal bloom made up of trillions of individual single-celled organisms dies, its demise is rapid and thorough. A team at the Weizmann Institute of Science found that viruses and bacteria conspire to kill off the algae time after time. As they recently reported in Science Advances, their study raises the intriguing possibility that a substance produced by the alga themselves plays a crucial role in the infection process.
Such blooms are so large they can be seen from space, and thus their rapid cycles of life and death have an outsized impact on everything from the marine food chain to the oxygen we breathe. Prof. Assaf Vardi of the Institute’s Plant and Environmental Sciences Department and his group investigate the mechanism by which a virus infects and kills the species of phytoplankton that make up these algal blooms. But such a virus would normally, over time, evolve less lethal relations with its host; or the host should, over successive cycles, evolve resistance. Since neither is the case in the demise of algal blooms, there was clearly more to the story than this one virus.
Vardi and a PhD student in his group, Noa Barak-Gavish, were looking for additional viruses that might attack phytoplankton, and they came up with the idea of looking in the “guts” of the next level up of the food chain – the zooplankton that eat phytoplankton, exclusively. By isolating the digestive contents of the zooplankton, Barak-Gavish and Miguel Frada, a former postdoc in Vardi’s group discovered that in addition to viruses, a strain of bacteria seemed to be present. These bacteria were ingested together with the phytoplankton, and lab experiments showed they could kill these microorganisms while they still floated in the ocean.

What role do these bacteria play in the death of an algal bloom? When Barak-Gavish grew the phytoplankton and bacteria together in the lab, they noted that in the initial stage both grew rapidly. At some point, however, things stalled. And then bacterial growth took off while the phytoplankton died. But the group noticed something else: The phytoplankton that were resistant to viral infection succumbed more easily to the bacterial one, and those that were more resistant to the bacteria were highly susceptible to the virus. “There was some sort of trade-off. Slightly different strains – even within the same bloom – responded in opposite ways to the same threats. A double attack – viral and bacterial – can finish off the bloom and prevent the next one from developing immunity,” says Vardi.
The bacteria belong to a family known as Sulfitobacter – that is, bacteria that specialize on sulfur metabolism. It just so happens that the phytoplankton produce a sulfur-containing compound that is generally known by its initials DMSP. Vardi, together with the Institute’s Prof. Dan Tawfik and their joint student Uria Alcolombri, had previously isolated and identified the enzyme that utilizes DMSP to produce a volatile compound, DMS. DMSP and DMS are responsible for many functions in the algal cells, among them a sort of “infochemical” that passes messages on to other neighboring cells. DMS is so prevalent, it is the “smell of the ocean,” and it can form new compounds in the atmosphere that then seed cloud formation.
DMSP, indeed, turned out to be key to the relationship between the bacteria and the phytoplankton. The algal strains that produced the most DMSP were also those that were most likely to succumb to bacterial infection. In fact, when the scientists added this sulfur compound to cultures of resistant phytoplankton, these lost much of their resistance. The bacterium eats DMSP and releases another compound – this one with the strong smell of ripe cheese. “As soon as I smell that pungent scent, I know our cultures will be dead within a day or two,” says Barak-Gavish.
In these cultures, the bacterial attack did not begin immediately; it took around a week for the originally symbiotic bacteria to turn pathogenic. This fits with another observation: The viruses tended to attack phytoplankton cells in their prime, while the bacteria affected the older plankton. Did the bacteria simply move in to feast on those that were not killed by the virus, or was something more complicated going on? Although they don’t yet completely understand the mechanism for the switch to pathogenicity, Vardi and Barak-Gavish think that DMSP might accumulate over time, making it easier for the bacteria to follow the “odor” trail to these organisms; bits of plankton exploding as they die from the virus may also attract them. The bacteria might then start producing phytoplankton-killing toxins out of the DMSP itself. Or they may respond to signals from other bacteria – of the same species or competing ones – or other signals indicating the phytoplankton are, anyway, near the point of natural death.
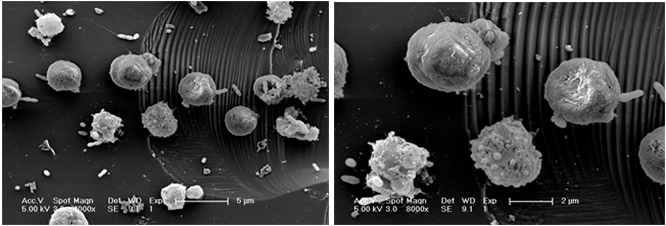
Among other things, the group intends to continue looking for clues in the samples taken from algal growths near the coast of Norway last summer – hundreds of frozen samples that represent a timeline of small, controlled blooms’ growth and demise. These will help the scientists determine whether the phenomena they witness in the lab occurs in the same way in the natural environment.
“The death of these blooms is the metabolic valve for the ocean’s food chain, and it plays a huge role in the planet’s oxygen, carbon and sulfur cycles,” says Vardi. “These global phenomena are regulated, to a large extent, by the trade-offs tiny microorganisms make between bacteria and viruses, and the interactions between these and the chemical produced by the phytoplankton for their own use. The trade-off may prevent the phytoplankton from evolving resistance to pathogens, and this, in turn, leads to the extreme cycles of algal growth and demise we see in the ocean.”
Also participating in this research were Drs. Chuan Ku and Sergey Malitsky, and Prof. Asaph Aharoni of the Institute’s Plant and Environmental Sciences Department, and Stefan J. Green from the University of Illinois at Chicago.
The death of these blooms is the metabolic valve for the ocean’s food chain, and it plays a huge role in the planet’s oxygen, carbon and sulfur cycles
Prof. Assaf Vardi's research is supported by the David and Fela Shapell Family Foundation INCPM Fund for Preclinical Studies; the De Botton Center for Marine Science; the Edmond de Rothschild Foundations; the Bernard and Norton Wolf Family Foundation; Scott Jordan and Gina Valdez; the estate of Emile Mimran; and the European Research Council.