Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

The last part of the brain to finish developing – sometime during adulthood – is the prefrontal cortex. This may be where, among other things, we learn to understand the needs and emotions of others; its dysfunction is believed to be central in autism spectrum disorder. Yet little is known about the way in which social information is encoded in this part of the brain. In a study recently published in Nature Neuroscience, a team at the Weizmann Institute of Science showed how neurons in the prefrontal cortex of mice distinguish between social and nonsocial stimuli, and they further revealed how the brains of “autistic” mice fail to properly distinguish between the two.
Mice, like humans, are highly social animals, and the ability to decipher complex social information is crucial to their survival. Researchers in the labs of Prof. Ofer Yizhar and Prof. Elad Schneidman in the Neurobiology Department decided to test the way mice process social information, using the animal’s sense of smell. “For mice, odors are a means of communication,” explains Dr. Dana Rubi Levy, who led the research together with PhD student Tal Tamir. “We exposed mice to different scents and measured how their brains respond to various odors through electrodes placed in their prefrontal cortex.” Some of the scents were of male or female mice. Others were the smell of peanut butter, which mice like; banana, which mice can take or leave; and the smell of fresh-cut grass, which they find slightly repellent.
The researchers found that the response in the prefrontal cortex was much stronger when the mice smelled other mice than when they were exposed to any of the other smells. The social responses involved around a quarter of the neurons in this area – more than twice as many as those responding to food or grass. “Preference for social smells was not seen in other parts of the brain that are responsible for processing odor information. It may be that this selectivity is confined to the prefrontal cortex,” says Prof. Yizhar.
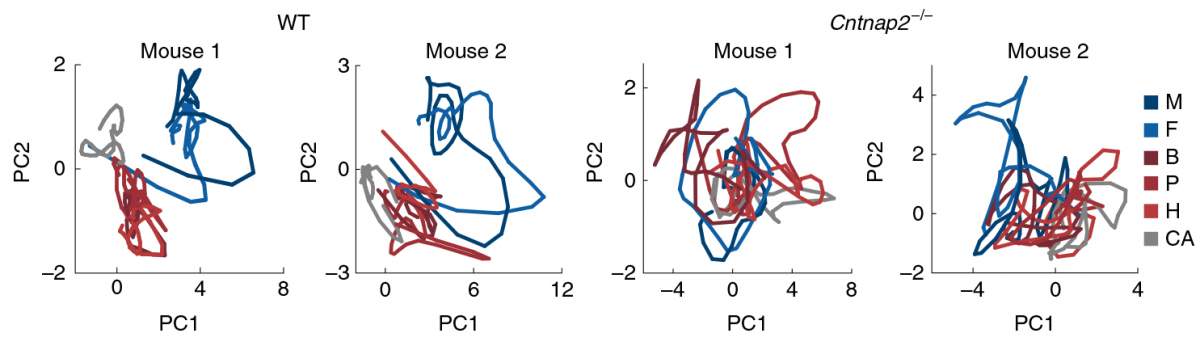
Just as we and mice are social, the neurons in our brains are also highly social – they have vast, complicated communication routes, and their language is encoded in shared electrical activity. Computational tools developed in Schneidman’s group for characterizing the joint activity patterns of networks of neurons enabled the researchers to uncover certain configurations that encoded each of the odors. Led by Tamir, the researchers were able to identify distinct population vocabularies that represented either social or nonsocial stimuli. “In this part of the brain, categorizing a smell as social or not is much more significant than whether it’s tempting, boring or repulsive,” says Tamir. And when the mice were exposed to these smells a second time, the category distinction was even more robust. So the mice were very quick to learn which was which.
Because people on the autism spectrum are known to have irregular activity in the prefrontal cortex as well as impaired social interaction, the researchers believe their findings could be relevant to understanding this disorder. The group repeated their experiments with mice that were genetically engineered to have mutations associated with autism. The neurons in the prefrontal cortex of these mice responded less to the social cues, and they hardly distinguished between the scents of other mice and the general, nonsocial, odors. In addition, their responses did not improve with repeated exposure; in fact there was little change. In contrast with the findings in the normal mice, the brain activity in this region in the mouse models of autism was quite “noisy”: There was much more spontaneous variability in neuron activity – that is, the communication between nerve cells had “interference” on the line.

“The noise we observed may be the key to the evident failure of the brains of these mice to create proper representations of social information, and thus to respond to social cues,” says Levy. The scientists think that in the future, finding ways to reduce the noise might, in some cases, lead to behavioral changes in those with autism spectrum disorder.
Prof. Elad Schneidman's research is supported by the Adelis Foundation. Prof. Schneidman is the incumbent of the Joseph and Bessie Feinberg Professorial Chair.
Prof. Ofer Yizhar's research is supported by the Ilse Katz Institute for Material Sciences and Magnetic Resonance Research; the Adelis Prize for Brain Research; Paul and Lucie Schwartz, Georges and Vera Gersen Laboratory; and Candice Appleton Family Trust; Prof. Yizhar is the incumbent of the Gertrude and Philip Nollman Career Development Chair.