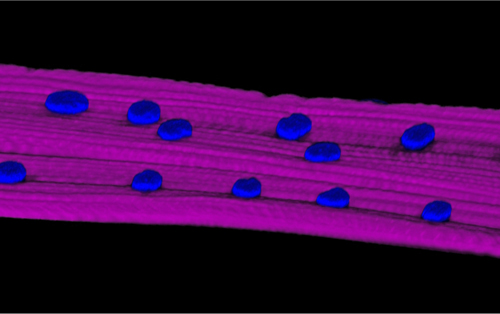
Everyone knows that exercise can build muscle; without exercise, muscles quickly lose their strength and volume. These facts have been common knowledge for so long, it might come as a surprise to find that very little is known about the molecular mechanisms behind them. How indeed does physical activity cause muscles to strengthen?

Prof. Talila Volk
Weizmann Institute scientists have now proposed an explanation. Research conducted by the team of
Prof. Talila Volk in the Molecular Genetics Department suggests that the long, cylinder-shaped muscle cells – the muscle fibers – contain proteins that function as biological “mechanosensors.” These spring-like, elastic proteins are connected to the cellular supporting structure – the cytoskeleton – on the one side, and on the other side, to the cell’s nucleus. Muscle contractions exert mechanical pressure on the cytoskeleton, which, in turn, puts pressure on these sensor proteins, causing them to transmit a signal to the nucleus.
This signal presumably alters the internal architecture of the chromosomes within the nucleus, changing gene expression – that is, the activity of genes – in the affected portion of the DNA. As a result, certain genes become activated, prompting the release of proteins that make up the filaments responsible for the contraction of the muscle fiber. In addition to contraction, the proteins fortify existing filaments and help produce new ones, building up muscle mass.
In this manner, exercise triggers a strengthening of the muscle. But because muscle-building proteins have a high turnover, the signal for their production must be periodically repeated. If, in the absence of exercise, the muscle fails to contract for a while, the mechanosensors no longer send their signals to the nucleus, ultimately leading to a loss of muscle mass.
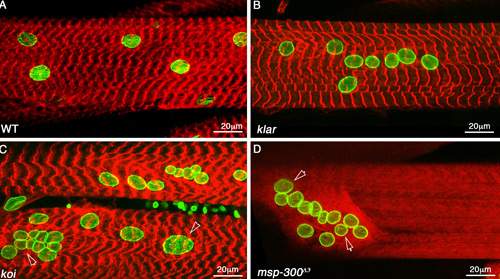
Muscle fibers of a fruit fly viewed under a confocal microscope: a normal fiber has normally-shaped, properly distributed nuclei (A), whereas the nuclei of fibers with mutated MSP-300 proteins are distorted and distributed abnormally (B, C and D)
Evidence for this explanation comes from a series of studies that Volk and her team – including research students Hadas Elhanany-Tamir and Miri Shnayder, and postdoctoral fellow Dr. Shuoshuo Wang – conducted in fruit flies.
As reported in the
Journal of Cell Biology, the scientists have identified a fruit fly protein, called MSP-300, whose shape and mechanical properties make it perfectly suited to serving as a mechanosensor. MSP-300 forms a ring around the nucleus, with numerous radial extensions connected to the cytoskeleton. It has elastic properties, as would be required from a mechanosensor; but on the other hand, it operates in close cooperation with two other proteins that help create rigid scaffolding around the nucleus, protecting it from muscle contractions. By introducing mutations into MSP-300, the scientists showed that it is indeed essential for maintaining muscle mass. The effect of the mutations on the fly’s muscles was devastating: The muscles fibers thinned out and their nuclei were deformed and clumped together abnormally. As a result, the muscles didn’t work properly, so the larvae couldn’t crawl and adult flies couldn’t fly.
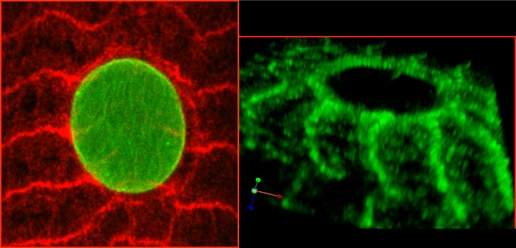
The MSP-300 protein, viewed here under a confocal microscope, forms a ring (red in the left image, green on the right) around the muscle cell nucleus, with extensions to the cytoskeleton
Because human muscle cells contain proteins equivalent to MSP-300, suggesting they may also function as mechanosensors, these findings can shed new light on the connection between physical activity and a healthy build-up of muscle in humans. A better understanding of this connection may in the future lead to improved ways to prevent muscle loss resulting from aging, forced inactivity as in paralysis, or such disorders as muscular dystrophy.
Prof. Talila Volk's research is supported by Erica A. Drake and Robert Drake. Prof. Volk is the incumbent of the Sir Ernst B. Chain Professorial Chair.