Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Our bodies contain cells that seem to be zombies – they no longer divide as they did in their prime, but they refuse to die. These “living-dead” cells, called senescent cells, take part in healing wounds and averting cancer, but as we age, they may do more harm than good. They tend to accumulate abnormally in various organs, contributing to such problems as osteoarthritis, a chronic inflammation of the joints, or atherosclerosis, a hardening of the arteries. Recent studies in mice suggest that clearing out senescent cells from the body may prevent age-related diseases and perhaps even increase lifespan.
“But if you want to kill senescent cells to alleviate or prevent disease, it’s important to know first of all how many such cells there are,” says Prof. Valery Krizhanovsky. He and his team in the Molecular Cell Biology Department of the Weizmann Institute of Science have developed a method for counting senescent cells in mice. The method, described in Aging Cell, may help devise ways of counting these cells in humans – to determine, for example, at what age they start accumulating and to evaluate the success of therapies aimed at eliminating them from the body.
We found several times more senescent cells in aged mice than we had expected
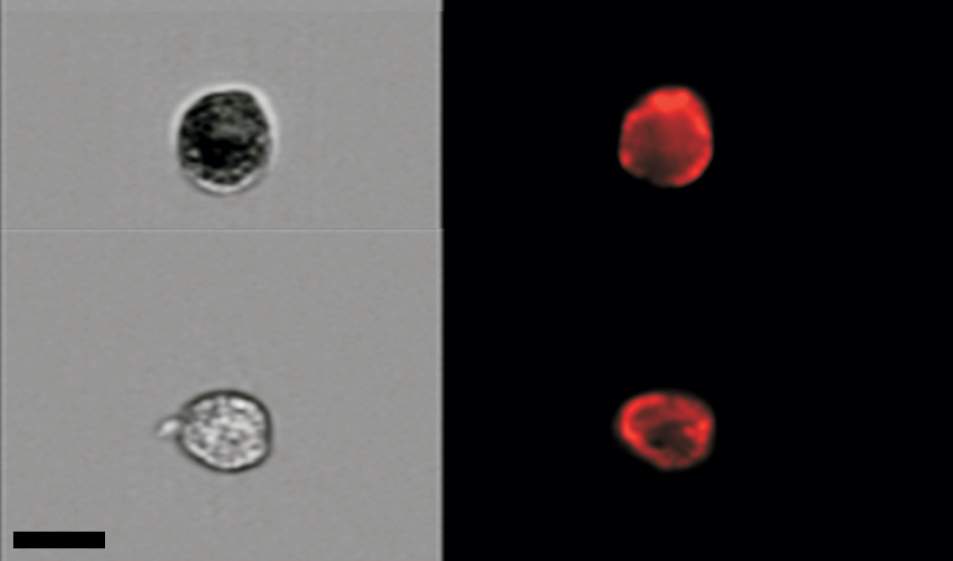
Krizhanovsky and his colleagues managed to count senescent cells in mouse tissue by combining two technologies. One consists of staining the tissue with a dye to highlight a desired molecular feature – in this case, cellular structures that identify these cells as senescent. The other, known as flow cytometry – implemented in collaboration with Dr. Ziv Porat of the Life Sciences Core Facilities Department – makes the count possible by spotting individual cells in a solution and creating high-resolution images of these cells. Using this combined approach, the scientists found that in the tissue of two-month-old mice, the proportion of senescent cells stood at below 1 percent. By contrast, in the bodies of two-year-old mice, it had soared to as much as 15 percent in certain organs. “We found several times more senescent cells in aged mice than we had expected,” says Krizhanovsky.

Yet another surprise came from an additional study that was reported recently in The EMBO Journal. Krizhanovsky and his team focused on one of the several mechanisms that usher cells into a senescent state: a molecular pathway in which a gene called p21 acts as a “brake,” preventing cells from dividing after their DNA has been damaged. This gene is activated, among other ways, by the tumor suppressor gene p53, so originally p21 was also thought to act as a tumor suppressor. If that were the case, mutated copies of p21 would have been found in cancerous tumors, while in fact, genomic studies have only rarely revealed such mutations.
Krizhanovsky and his colleagues wanted to find out how exactly p21 keeps cells in a state of senescence. When they turned down the activity of p21, they expected that in the wake of this genetic manipulation – known as a “knockdown” – senescent cells would start dividing again, at least for a few cycles. That was precisely when the surprise appeared: Rather than dividing, the senescent cells started dying.
Apparently, without sufficient p21 activity, these cells went into an overdrive of sorts: They rapidly accumulated so much DNA damage that they quickly died. “When we loosened the ‘brake’ applied by the p21 gene, these cells galloped forward too fast, speeding their own death,” Krizhanovsky says.
Silencing p21 may in the future prove a promising strategy for eliminating senescent cells
Using a mouse model, the scientists then investigated the involvement of p21 in liver fibrosis, in which senescent cells accumulate in large numbers in the liver, contributing to the formation of scar tissue. They found that in mice lacking the p21 gene, the number of senescent cells in the liver was lower than in regular mice, and their diagnostic markers associated with fibrosis improved.
The study suggests that silencing p21 may in the future prove a promising strategy for eliminating senescent cells to keep body tissues healthy and delay the ravages of aging.
The research team included Dr. Hilah Gal, Dr. Anat Biran, Dr. Reut Yosef, Dr. Noam Pilpel, Dr. Yossi Ovadya, Nurit Papismadov, Lior Roitman, Lior Zada, Paula Abou Karam, Stav Miller and Ezra Vadai, all of the Molecular Cell Biology Department, and Dr. Shifra Ben-Dor of the Life Sciences Core Facilities Department.
Prof. Valery Krizhanovsky's research is supported by the Rising Tide Foundation; Mr. and Mrs. Bruce Kanter; and the European Research Council.