Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Shaped through time, biological cells are the ultimate engineering systems, able to perform the most advanced information processing known. They also produce a wonderland of materials - from over 100,000 proteins to the materials they help build: skin, record-strong spiderwebs, horns and far more. The cell pulls off these feats in a tiny setting that engineers can only dream of. How do its systems work? Might they be harnessed to build superfast computers or advance new biotechnologies?
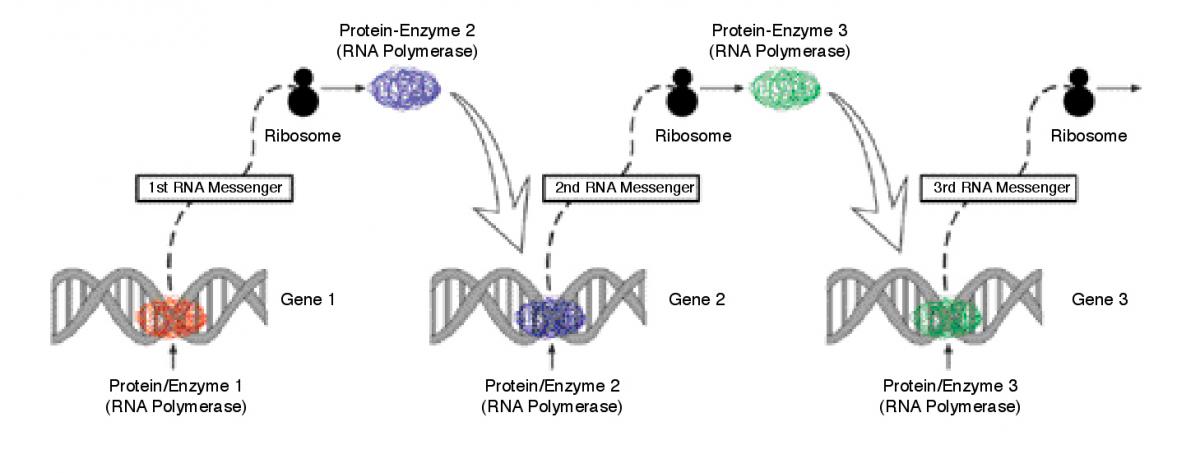
In a step that might help address these questions, a Weizmann scientist has now designed the first synthetic circuit able to process genetic input to produce proteins. The circuit works on the principles of a conventional electrical circuit - that of a flashlight, for instance - but is constructed entirely of genes, proteins and other biological molecules. “Our goal was to determine whether an assembly of these components could be made to operate outside the context of a living cell,” says Dr. Roy Bar-Ziv, of the Institute’s Materials and Interfaces Department, who performed the work with Prof. Albert Libchaber and Dr. Vincent Noireaux of Rockefeller University in New York.
The circuit built by the scientists floats in a biochemical “soup” containing the necessary ingredients and machinery for processing genetic information to produce proteins. The circuit inputs are genes (DNA molecules coding for proteins) which are “wired-up” so that the protein encoded by one gene can either activate or depress the production of neighboring proteins. The circuit design also uses an external sugar molecule that, functioning much like a biochemical switch, turns on the protein synthesis.
While other scientists have developed single-gene systems, this is the first time researchers have rigged up a multiple-gene circuit outside the cell. Though rudimentary, this synthetic circuit offers an isolated and thus highly controllable environment in which to explore the workings of the cell. Moreover, it may represent the first step to streamlined protein production plants or advanced biocomputers. Unlike conventional computer systems, in which information is processed through a rigid digital 0-or-1, yes-or-no framework, biological networks are able to plod toward their goal using the multi-branched routes characteristic of parallel processing. This inherent property, researchers believe, might significantly fast-forward computer processing.
But this won’t happen any time soon. The system’s DNA-to-protein reactions can take an hour or more, and it takes time until enough of the first material is produced to initiate the next stage. When too many stages are added to the sequence, the reactions tend to fizzle out as available resources are used up.
The next step, says Bar-Ziv, is to try to introduce circuitries of this sort into different materials. Once it is possible to create positive and negative feedback systems to turn things on and off, one could potentially design artificial circuits that mimic transistors, sensors, memory elements and clocks. “The gene is hardware and software all rolled up into one, and we need to learn to work with its unique properties,” says Bar-Ziv. “Scientists are busy trying to invent self-replicating nanotechnology, but why not use what already exists?”
A different type of building
Dr. Bar-Ziv credits his current career track to his background in both theoretical and experimental physics (through M.Sc. and Ph.D. degrees completed at the Institute under Profs. Sam Safran and Elisha Moses, respectively), followed by a 3-year postdoc at Rockefeller University under Prof. Libchaber, where he focused on biological systems. The lab, says Bar-Ziv, is the best kind of work/playground he can imagine - where he can combine his fascination with the abstract questions of physics with his dream of building artificial biological circuits.
* Prof. Libchaber received an honorary doctorate from the Weizmann Institute in 2003.
Dr. Bar Ziv’s research is supported by the Clore Center for Biological Physics; the Sir Charles Clore Prize - the Clore Foundation; Sir Harry Djanogly, CBE, U.K.; the Philip M. Klutznick Fund for Research; the Levy-Markus Foundation; and the Lord Sieff of Brimpton Memorial Fund. He is the incumbent of the Beracha Foundation Career Development Chair.