Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
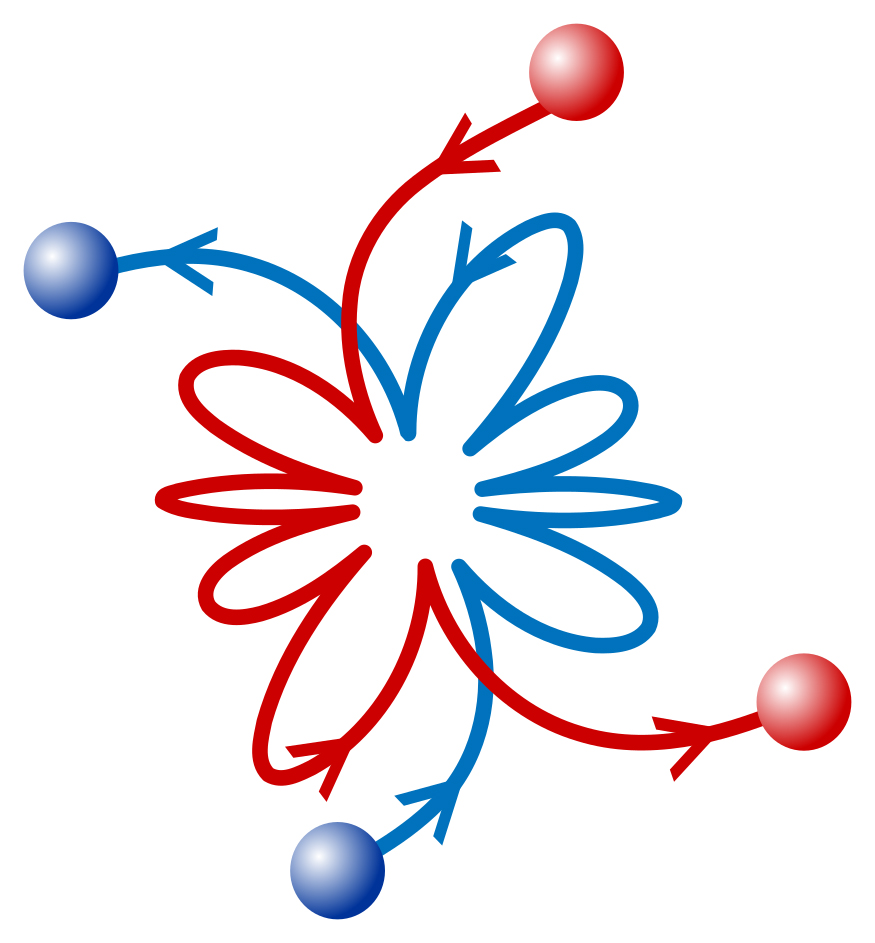
When two particles collide, they normally fly off in opposite directions, like billiard balls on a smooth table. But in a recent physics experiment at the Weizmann Institute of Science, instead of flying off, the particles seemed to engage in a sort of a post-collision billiard-ball ballet. The two – one an atom and the other an ion, an atom with an electric charge – acted as though they were connected to one another by a long, invisible spring, coming closer and then moving apart in a repeated pas de deux.

“We first discovered this surprising interaction between an atom and an ion in computer simulations, but we only became convinced it was real after observing it in actual experiments,” says PhD student Meirav Pinkas, who led this research project in Prof. Roee Ozeri’s lab in Weizmann’s Physics of Complex Systems Department.
Pinkas explains that the original goal of this experiment was to observe new quantum effects. Many previous experiments had examined quantum effects in ultracold collisions among atoms or among ions, but very few had paired atoms and ions, as exploring interactions between an atom and an ion is technically quite challenging. Yet such interactions are of great interest, in part because they shed light on chemistry at very low temperatures, the kind that occurs, for example, in interstellar space. Ozeri’s lab is well equipped to rise to the challenge thanks to its long-standing experience working with both ultracold atoms and ultracold ions.
""We were looking for quantum effects and found a classical physics effect we didn’t expect"
The experiment designed by Pinkas and the team was performed in ultracold conditions: at temperatures below one millikelvin, or one thousandth of a degree above absolute zero. These conditions were created by laser cooling: Counterintuitive as this may seem, the laser cools the atoms, or ions, by hitting them until they are nearly frozen in place. Once the setup is chilled, a single ion of the metal strontium, trapped by means of an electric field, is exposed to a flow of about half a million atoms of rubidium. When the strontium ion collides with one of the rubidium atoms, the scientists can detect the outcome of the collision by means of a second laser.
Generally, the cooler the system, the greater the chance to see quantum phenomena, which are characterized by discrete energy levels; these become lost in the more chaotic particle motion that comes with the warming. The quantum threshold for the system used in the present experiment usually stands at about a tenth of a millionth of a degree above absolute zero.
But the new discovery was made before this ultracold state was reached, so the experimental setup was still relatively “warm”: The temperature first stood at one millionth of a degree above absolute zero, then rose to one thousandth of a degree, driven upward by the use of the ion trap. This was much too warm for quantum physics observations, but it was classical physics that supplied the surprise; in fact, the peculiar dance of the ion-atom pair observed in the experiment could be entirely explained by Newtonian equations.
“We were looking for quantum effects and found a classical physics effect we didn’t expect,” Pinkas says.
The researchers could tell that something unusual was going on after checking the strontium ion’s quantum property called spin. After the collision, the spin kept changing, or in scientific jargon, “flipping,” at a much higher rate than predicted, which suggested that, contrary to expectations, the ion remained somehow connected to the rubidium atom. The scientists then realized that the atom and the ion, in the interconnected state that emerged from their collision, formed a molecule of sorts – short-lived and characterized by an unusually large distance between the two components, but a formation that behaved as a molecule, nonetheless.

“This wasn’t supposed to happen, because two atoms that form a molecule lose energy in the process, and in our system that energy seemingly had nowhere to go,” Pinkas explains. “But we found an explanation. The surplus energy is absorbed by the ion trap, which for a short while prevents the atom and the ion from parting ways after a collision. In the billiard analogy, it’s as if the sides of the pool table curved upward to resemble a bowl, temporarily keeping the two colliding balls from flying off.”
The team – which also included Dr. Or Katz, Jonathan Wengrowicz and Nitzan Akerman – continued to apply quantum tools to explore the strontium-rubidium molecule they had created. For example, by changing the strength of the magnetic field in the ion trap, they could alter the particles’ spin and ask how this would affect the molecule’s formation. The ultimate goal is to understand not just how the molecule comes into being, but how it can be broken apart. “We want to learn to gain precise control over the effect we discovered,” Pinkas says.
Achieving such control could help transfer the new findings into the quantum domain. It might, for example, make it possible to produce quantum effects at higher temperatures than today, which would be useful for the study of quantum chemistry, including chemical reactions that take place in the ultracold environment of interstellar space. In fact, the most prevalent chemical reaction in interstellar space is the formation of molecular ions through cold collisions between atoms and ions. Another potential future direction is to use ion-atom interactions to build certain physical models that are difficult to produce otherwise, for example, models of crystals. In these, the ion would mimic the atomic nucleus and the atoms, the behavior of electrons surrounding this nucleus. Such models could be used in materials science or in fundamental studies of matter.
Adds Ozeri: “These are potential directions we can envisage today, but the beauty of all new discoveries is that they might lead us into entirely uncharted territory in ways that at present we cannot even imagine.”
The ion-atom molecules created in the experiment existed, on average, for 1 microsecond (1 millionth of a second).
When the ion and the atom bounced off one another about 5 times after the collision in the Weizmann Institute experiment, the distance between them was about 30 nanometers. By contrast, in regular molecules held together by chemical bonds, the distance between the atoms is less than 1 angstrom, or 0.1 nanometers.