Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

A brilliant flash of lightning streaks across the evening sky followed by a sharp crack of thunder. As you rush upstairs to close the bedroom window, the millions of cells making up your heart muscle continue their endless task of contracting and relaxing in concert, guided by a small group of conductor cells. You then turn up the heater, relishing the wave of hot air as it meets your skin.
Electricity is everywhere: in nature, appliances and the human body. A steady heartbeat, mental processing, the perception of sounds, sights and temperatures - all depend on meticulously orchestrated cellular communication pathways linked up through electrochemical signaling.
One of the body’s primary communication pathways consists of a tiny pipe located in the cell membrane that opens to allow ions (electrically charged atoms) to flow into or out of the cells according to specific signals. These pipes, known as ion channels, are “motivated” to open by electrical differences existing between a cell’s internal and external environment.
Cells maintain an electric charge that is negative relative to their external environment. When these channels open, this difference in energy propels positively charged ions through the channel, triggering a variety of cellular processes.
Most channels allow the passage of only one type of ion. Yet how do different channels open and close? What enables them to act like bouncers at a club, selectively determining which ions (such as sodium, calcium or potassium) will enter or exit the cell?
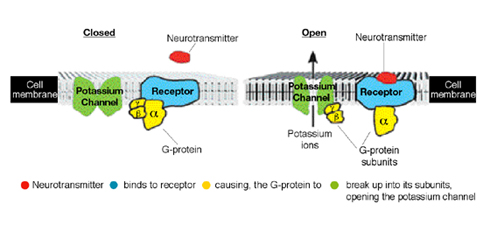
Prof. Eitan Reuveny of the Institute’s Biological Chemistry Department is studying this cellular feat in potassium channels - key ion channels affecting the electrical state of the heart, nerves and muscles.
Previous research had revealed that the potassium channel contains a selectivity filter that identifies and binds to potassium ions, filtering these ions from the intracellular solution. It does so with remarkable speed - tens of millions of ions are identified and travel through a single channel every second. The channel opens when an intracellular molecule, called a G protein, is activated, causing four of its subunits to rearrange themselves, thus permitting ion flow. The mechanism is similar to that of a door latch. Following activation, the G protein subunits bind to the channel, changing its formation in a way that essentially presses down a handle, pulling a door tongue inside and thus opening the channel.
To probe this “door-latch” mechanism, Reuveny is combining exciting new technologies from the worlds of biophysics and molecular genetics to do what was unthinkable just a few years ago: identify the tiny fluctuations of electric potential that occur within an individual cell. One of the technologies applied (which earned its developers, German cell physiologists Erwin Neher and Bert Sakmann, the 1991 Nobel Prize in Physiology or Medicine) is based on using microscopic glass pipettes a thousandth of a millimeter in diameter. By sucking in a tiny part of the cell membrane containing only one ion channel, the pipettes make it possible to measure the incredibly tiny current created as ions pass through.
Working with graduate students Rona Sadja, Karin Smadja, Noga Alagem and Inbal Riven, Reuveny began by introducing genetic modifications that in effect shortened the latch tongue, causing the channel to open even without G protein activation. Next, they tagged the channel with “reporter” proteins that lit up under certain optical conditions, making it possible to trace the actual movements of the channel latch.
Using this double strategy, the team succeeded in identifying the key molecular elements that open the potassium channel. Later research showed that these same elements also fulfill an important role in stabilizing the channel once it opens. The team’s findings were published in Neuron.
A better understanding of the rules governing potassium and other ion channels will clarify some of the most basic life processes. Insights into what goes wrong when cellular communication pathways break down may also lead to new therapies, from those targeting heart arrhythmia to diabetes and a range of neuronal disorders.
In related research, Prof. Reuveny is studying how fluctuations in the electrical activity of potassium channels trigger a “shut-down” response that controls insulin release. Research in this field might lead to a new therapy for hypoglycemia - a complication of Type 1 diabetes occurring when elevated levels of insulin flow into the blood, causing glucose to drop to dangerously low levels.
Prof. Reuveny’s research is supported by the Y. Leon Benoziyo Institute for Molecular Medicine; the Clore Center for Biological Physics; the Dr. Josef Cohn Minerva Center for Biomembrane Research; and the Buddy Taub Foundation.