Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Molecules come in all shapes and sizes. Directly imaging molecules is a feat that leads to advances in fields ranging from medicine to physics, so researchers have been taking up this challenge for over a century. An early leader in determining molecular structure was Max von Laue, who in 1912 had the idea of bombarding molecular crystals with X-rays and measuring how the rays are scattered from the crystalline structure or how they pass through it. The 1914 Nobel Prize in Physics was awarded to von Laue for this achievement.
Nearly 100 years later, in 2009, Prof. Ada Yonath of the Weizmann Institute of Science would receive a Nobel Prize in Chemistry, together with Thomas Steitz and Venkatraman Ramakrishnan, for solving the structure of the ribosome. Yonath overcame a major challenge facing X-ray crystallographers – namely the need to crystallize such large, intricate molecules as proteins or even ribosomes (complexes made of proteins and RNA) so as to determine their structure.
More recently, last year’s Nobel Prize in Chemistry, awarded to Jacques Dubochet, Joachim Frank and Richard Henderson, recognized another advance in imaging: cryo-electron-microscopy, in which electrons, rather than X-rays, are the observation agents. This type of microscope was recently installed at the Weizmann Institute.

The next step on the road to an efficient method of solving molecular structures might involve measuring scattered, ultrashort X-ray pulses from non-crystallized molecules. Doing away with the need for crystallization could immensely speed up the process of solving many molecular or molecular-complex structures and make it more accessible. However, free molecules point in all directions; if they also scatter the pulses in different directions, the process of solving their structure from this information would be quite difficult. To avoid this complication, researchers need to order the molecules in a sample, getting them to “stand in formation.” One way to do this would be with laser pulses that are very short and very fast – so fast that they beat the speed of some internal processes taking place in a molecule.
In a paper recently published in Nature Communications, Profs. Ilya Averbukh and Yehiam Prior and research student Ilia Tutunnikov of the Weizmann Institute of Science’s Chemical and Biological Physics Department, together with Prof. Jian Wu from Shanghai’s East China Normal University and his team, showed how lasers can be used to line up the selected molecules in the desired direction. Although the demonstration molecule was a small one, the idea should work on large, complex molecules as well. Once the lasers have the molecules aligned in a particular orientation, the same laser pulses would be used to generate the short X-ray pulses that scatter from the molecules and provide the image.

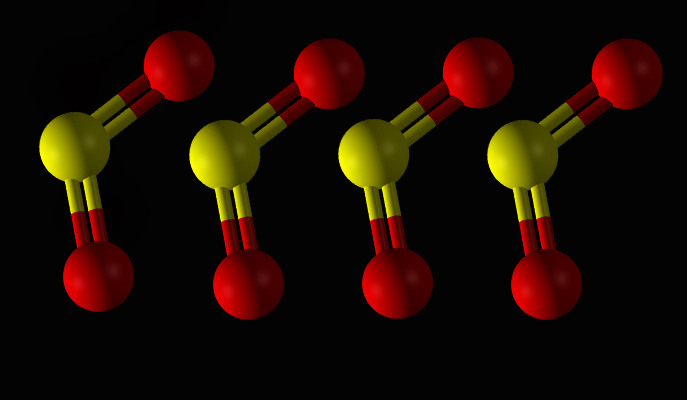
The molecule used for the demonstration, sulfur dioxide, is made of two atoms of oxygen and one of sulfur. These two oxygen atoms create an axis for the molecule, with the sulfur freely rotating around this axis. “To fix the molecules in space, we used two overlapping laser pulses,” says Prof. Prior. “The laser pulses were tuned so that the first one aligns the oxygen axis while the second pulse, which was carefully coordinated and synchronized with the first one, caused the sulfur atoms to line up in a perpendicular direction. The process involved the fine control of the pulses’ duration, their intensity and relative phases. Ultimately, with just two finely tuned laser pulses, we were able to stabilize the sulfur dioxide molecules and cause them to adopt the same orientation.”
Because the method does not rely on “freezing” the molecules into a crystal, it may not only advance the study of the 3-D structure of various complex molecules, but could also open new possibilities for researching the time-dependent behavior of these molecules. Molecular “movies,” say the scientists, are just around the corner.
Prof. Ilya Averbukh is the incumbent of the Patricia Elman Bildner Professorial Chair of Solid State Chemistry.
Prof. Yehiam Prior's research is supported by the Centre National de la Recherche Scientifique.