Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
More than a century ago, after discovering that living systems have a preference for either right- or left-handed molecules, Louis Pasteur suggested it might be possible to alter molecular “handedness” by exposure to a magnetic field. Lord Kelvin, on the other hand, believed that magnetism had nothing to do with handedness, for which he had coined the scientific term “chirality.” In a study published recently in Science, researchers from the Weizmann Institute of Science and the Hebrew University of Jerusalem resolved the argument over magnetism and chirality – or, as they put it, “a century-old argument between two giants of science”: It had been Pasteur who was on the right track, after all. The finding has led to a new method for separating molecules according to their chirality, an issue of immense importance in the pharmaceutical and chemical industries.
Chiral molecules have identical structures except that they are mirror images of one another, like the right and left hand. They may look the same, but molecules of opposite chirality can perform entirely different functions. For example, a molecule with one type of chirality may serve as a drug whereas one with mirror-image chirality may cause unwanted side effects. Since most chemical reactions randomly produce both right- and left-handed molecules, drug manufacture requires additional steps in which these molecules are sorted by chirality.
It had been Pasteur who was on the right track, after all
Current methods used for separating out the molecules with the desired chirality are complex and expensive; they rely on equipment that has to be tailored anew to each substance. Prof. Ron Naaman from Weizmann’s Chemical and Biological Physics Department and Prof. Yossi Paltiel from the Hebrew University, together with colleagues, have invented a generic method for sorting out the chiral molecules of any substance simply and cheaply.
The scientists took advantage of the fact that chirality exerts an effect on the property of electrons known as spin – angular momentum that can be either “spin up” or “spin down,” akin to the clockwise or counterclockwise rotation of a spinning top. When chiral molecules interact with a surface, they become polarized due to the movement of electrons from one side of the molecule to the other. In the course of this movement, electrons behave as if a magnetic field is acting upon them: Electrons with the “spin up” gather on one side of the molecule and those with the “spin down” on the other. Which spin lands on which side depends on the chirality of the molecule, that is, on whether it is right- or left-handed. This phenomenon, discovered previously by Naaman’s group, is known as chirality-induced spin selectivity, or CISS, for short. It means that chiral molecules can act as spin filters.
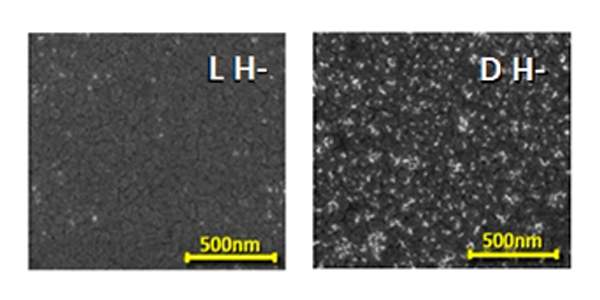
The scientists proposed exploiting CISS to separate out a substance by chirality. They introduced a magnetic surface – in which the spins of all electrons are aligned in the same direction – into a solution containing right- and left-handed molecules. When the molecules approached the magnetic surface, they became polarized as the electrons moved up or down within them, and the spins of the electrons on each pole arranged themselves in accordance with each molecule’s handedness. Some were the same as the spins of the electrons in the magnetic surface, others were opposite. Only those molecules that had opposite spins – and thus one particular chirality – stuck to the magnetic surface; those in which the spins were the same, were repelled. The interaction with the magnetic surface therefore made it possible to separate the molecules by their handedness, vindicating Pasteur in his belief that physical forces acting on molecules, such as those exerting their effect in a magnetic field, may be related to chirality.
This method can be used to purify drugs by chirality, leaving only therapeutic molecules while removing ones that are unsafe. In addition, it can be used to produce highly effective chemicals such as fertilizers and pesticides. In these chemicals, only molecules of a particular chirality produce the desired effect, whereas those with the opposite chirality are useless, causing nothing but pollution of the soil. By separating out molecules by chirality, it may be possible to create fertilizers and pesticides with increased efficiency, cutting down on costs while at the same time significantly reducing pollution.
This method can be used to purify drugs by chirality, leaving only therapeutic molecules while removing ones that are unsafe
Prof. Naaman’s team included Drs. Koyel Banerjee-Ghosh, Francesco Tassinari and Eyal Capua from the Chemical and Biological Physics Department; Prof. Paltiel’s team included Drs. Oren Ben Dor, Shira Yochelis and Amir Capua. Also contributing to the study were Dr. See-Hun Yang and Prof. Stuart S. P. Parkin from the IBM Research Division in San Jose, CA; Dr. Soumyajit Sarkar and Prof. Leeor Kronik from Weizmann’s Materials and Interfaces Department; and Prof. Lech Tomasz Baczewski from the Institute of Physics, Polish Academy of Sciences.
Prof. Ron Naaman's research is supported by the Nancy and Stephen Grand Research Center for Sensors and Security; the Rothschild Caesarea Foundation; the Weston Nanophysics Challenge Fund; the John Templeton Foundation; and the European Research Council. Prof. Naaman is the incumbent of the Aryeh and Mintzi Katzman Professorial Chair.