Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
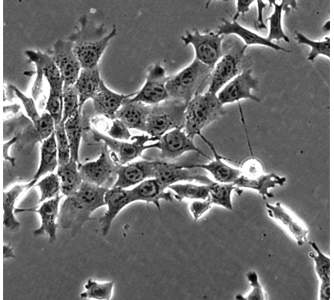
Invasive cancer cells – the ones that may lead to deadly metastasis – are said to be divided into two types: those that venture out, across vessel walls, into the bloodstream alone; and those that travel in groups. Until now, it was thought that the lone travelers were the most dangerous, as they are able to migrate through connective tissues throughout the body and infiltrate the blood vessels and lymphatics. However, researchers at the Weizmann Institute of Science recently revealed a “middle way,” in which cells can travel in groups yet exhibit the aggressive behavior normally associated with single-cell migration. This new understanding of cancer cell invasion, says Prof. Benjamin Geiger of the Institute’s Molecular Cell Biology Department, may help solve the puzzle of certain metastatic cancers − for example, some breast cancers in which the migration does not proceed according to the prevailing assumptions − and it may lead to new ideas for preventing metastasis. These findings were recently published in Scientific Reports.
Cancer cell invasion involves a sort of identity change. Often epithelial cells – largely stationary cells that line the milk ducts or intestinal wall – transform into migratory cells that can travel through tissues and across vessel walls. As part of this transition, the levels of a protein called E-cadherin, which is normally involved in the formation of bonds that hold epithelial cells in place, drop dramatically. The lone migrating cells are believed to undergo a nearly complete transformation that leaves them with very little E-cadherin, so they can make a clean break and enter the bloodstream, which carries them to distant organs. Those that travel in groups, in contrast, are thought to undergo a partial transformation, in which E-cadherin does not completely disappear, and to move as a cluster only a short distance from the main tumor.
Geiger and his graduate student Yair Elisha investigated a type of breast cancer cell that is aggressive in metastasis. They first placed the cancer cells in lab dishes in a sparse formation that would enable the cells to divide and to communicate without pressure, and they followed their development with time-lapse microscopic videos. The videos showed that when these cells reached the migration stage, they began pulling away from one another. But the detachment was incomplete: Instead, the cells stayed connected through long stringy “tethers” that gave each cell a fair amount of freedom of movement yet kept them tied together. Geiger says the cells looked something like a dog walker’s scene in which the leashes become tangled. Each can pull in a separate direction, yet each spurs the others onward and they end up moving as a group.
The scientists found that these cells had around half the normal levels of E-cadherin, which apparently allowed considerable individual freedom and at the same time ensured collectivity. To probe the tether formation and its connection to E-cadherin, the researchers conducted a series of experiments in lab dishes and in mice. Indeed, they found that higher levels of the protein keep cells closely attached to one another, while very low levels enable cells to completely break free. But when levels are “just right” in the middle, the tethers can form.
Perhaps more surprising was their finding that the tethered groups of cells were more metastatic − that is, they developed new cancerous growth in a secondary, often distant organ. As expected, when the scientists blocked E-cadherin production in the cells, tethers did not form and the cells arrived at the lungs or other organs one by one. Despite the fact that lone cells moved faster than the tethered ones, they were less efficient when it came to invasion or developing metastases.
Although it is still not clear exactly how the tethers are regulated, Geiger says the findings of this study are already overturning some ideas about how metastasis begins. In addition to confirming the role of E-cadherin in this process, which could point to new diagnostic tools and treatment targets for certain cancers, they suggest that the transformation from one cell type to another is not a rigid requirement, but rather a sort of wide range in which the cells can operate. Cells that travel in tethered formation and stay closer to their original cell type might expedite the migration process. Geiger believes that the tethers, themselves, may also play a role in helping the cancer cells colonize new tissue, and he and his group are continuing to investigate this possibility.
Prof. Benjamin Geiger's research is supported by the de Picciotto-Lesser Cancer Cell Observatory In memory of Wolf and Ruth Lesser; the Leona M. and Harry B. Helmsley Charitable Trust; David and Molly Bloom, Canada; and the European Research Council. Prof Geiger is the incumbent of the Professor Erwin Neter Professorial Chair of Cell and Tumor Biology.