Without collagen, we would all go to pieces – quite literally. This large molecule, the most abundant protein in our bodies, is the “glue” that holds tissues together. In fact, in our distant evolutionary past, it was collagen that enabled our single-celled ancestors to evolve into multi-celled creatures – by helping individual cells stick together.
During embryonic development, large amounts of collagen – the starting material for bone formation – are produced in the growing bone. Since collagen production is a complex, multi-stage process that requires a great deal of oxygen, one would expect to see a massive supply of oxygen to this developing body part.
Yet paradoxically, the growing bone is exceptionally low in oxygen: As an internal organ, it receives less oxygen than external embryonic tissues. In fact, oxygen-carrying blood vessels are actively pushed out of the cartilage that builds up in preparation for bone formation.
A study conducted at the Weizmann Institute of Science and published in
Development sheds light on this paradox. Though it remains unknown why so little oxygen is available during bone formation, the Weizmann findings explain how collagen production can take place in such seemingly unfavorable conditions.
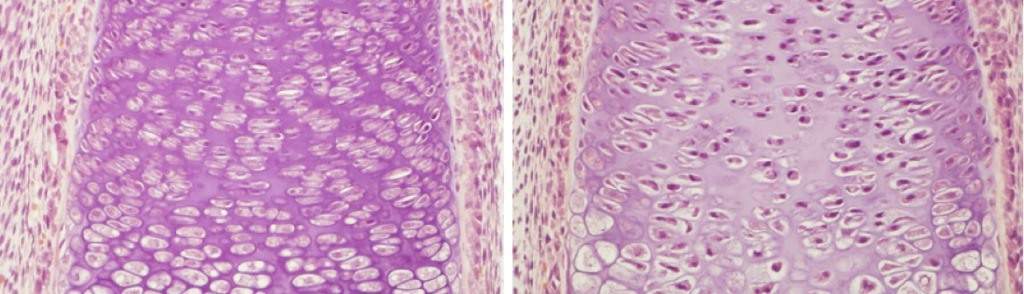
Prof. Elazar Zelzer, Lital Bentovim and then grauate student Dr. Roy Amarilio, all of the Molecular Genetics Department, investigated collagen formation in the growing bones of mouse embryos, focusing on a molecule, HIF1-alpha, which is known to regulate the cellular and physiological responses to low-oxygen conditions in tissues. The scientists discovered that HIF1-alpha also serves as a central regulator of collagen formation and renders the process highly efficient, so as to make the most of the available oxygen. When they blocked the action of this molecule, collagen release was impaired and the bone did not grow properly.
The scientists found that like a capable manager, HIF1-alpha creates optimal conditions for the collagen production. First it increases the release of catalytic enzymes that speed up the process. Then it shuts down other metabolic processes in the tissue, so that the little oxygen available can be channeled in its entirety to the collagen-secreting cells, enabling them to focus exclusively on their main task.
In addition to clarifying the formation in the embryo of one of the body’s central building blocks, this study may contribute to our understanding of disease. It may, for example, help explain how certain cancers develop despite low oxygen levels in the malignant tissue.
Attached
Bones, muscles and tendons give the body form, keep it stable and enable it to move. But for these functions to be successfully performed, they must be assembled into a single, precisely regulated system – the musculoskeletal system. A another study conducted in Zelzer's lab, reported in
Development, has revealed a
crucial mechanism responsible for this assembly.
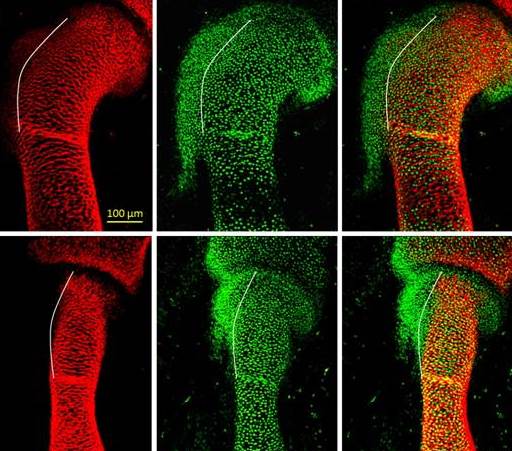
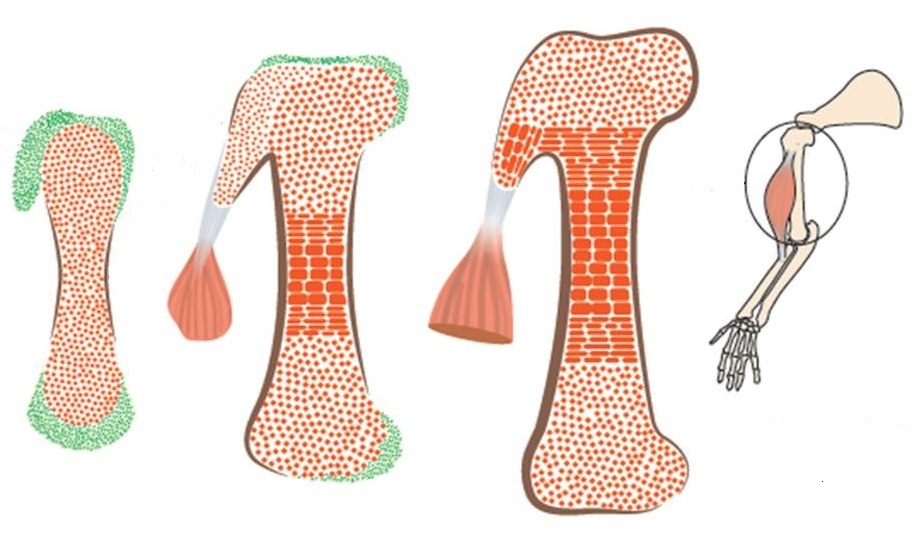
A fundamental step in the assembly process is the development of attachment units between a bone and a tendon – protrusions of various shapes and sizes known in technical language as bone eminences, which grow on the surface of the bone. These protrusions are vital for the proper functioning of the musculoskeletal system: They provide stable anchoring points for muscles that are inserted into the bone via tendons, as well as helping dissipate the stress exerted on the bone by contracting muscles.
In the new study, conducted in mouse embryos, the scientists have discovered that the bone protrusions are formed by a distinct, previously unknown class of cells that differ from the regular bone-forming cells. These protrusion-forming cells have a split personality of sorts: They are controlled simultaneously by two genetic programs – one characteristic of bone, the other of ligaments and tendons. This dual nature is what facilitates the attachment of the tendons, ligaments and muscles to the bone protrusions.
The scientists were able to establish the details of the genetic programs, including the molecular signaling that regulates them, by creating mutant mouse embryos lacking certain genes and tracing the embryonic development. The research was performed by Zelzer and then graduate student Dr. Einat Blitz.
By revealing that bones are created in the embryo in a modular fashion, the study might help explain their mechanical properties – for example, the ability of different anatomic regions of bone to cope differently with stress and load, and their contribution to the skeleton’s overall sturdiness and flexibility.
Dr. Elazar Zelzer’s research is supported by the Y. Leon Benoziyo Institute for Molecular Medicine; the Jeanne and Joseph Nissim Foundation for Life Sciences Research; the Helen and Martin Kimmel Institute for Stem Cell Research; the Irving and Dorothy Rom Charitable Trust and the estate of David Levinson.

.jpg)