Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Chronic heart disease and heart failure affect as many as 10% of adults over 65. And there is still no way to regenerate heart tissue after it stiffens and gets replaced with excess scar tissue, gradually reducing its ability to pump blood around the body. Researchers at the Weizmann Institute of Science have now shown, in mice, exactly how the heart can regenerate, through activating cells in the remaining heart tissue – it just needs the right kind of prompting.

After birth, human heart muscle cells – cardiomyocytes – are set for life. They almost never divide, regenerate or engage in repair when there is damage. In previous research, scientists in Prof. Eldad Tzahor’s lab in the Institute’s Molecular Cell Biology Department had discovered that newborn mice can still regenerate their hearts by activating a receptor on the cardiomyocytes, called ERBB2, which is essential for embryonic heart development. When the scientists caused this receptor to be activated on the membranes of adult mouse cardiomyocytes immediately following a heart attack, they found their hearts could reverse the damage. In the new study, Alla Aharonov, a research student in Tzahor’s lab, set out to further investigate this seemly miraculous occurrence, as well as to ask whether the same receptor could be activated at a later stage – when the heart has already undergone the structural changes that characterize heart failure.
The experiments were conducted with transgenic mice that were engineered to overproduce ERBB2 specifically in cardiomyocytes. The engineering included a “key” for activating these receptors on demand, giving the researchers a way to precisely control the timing of the regeneration process. The mice underwent heart attacks and the researchers let the situation progress to severe heart disease before using that key to switch on the ERBB2 receptor. The ERBB2 signaling in those cardiomyocytes did something remarkable – these cells divided and formed brand-new heart tissue.
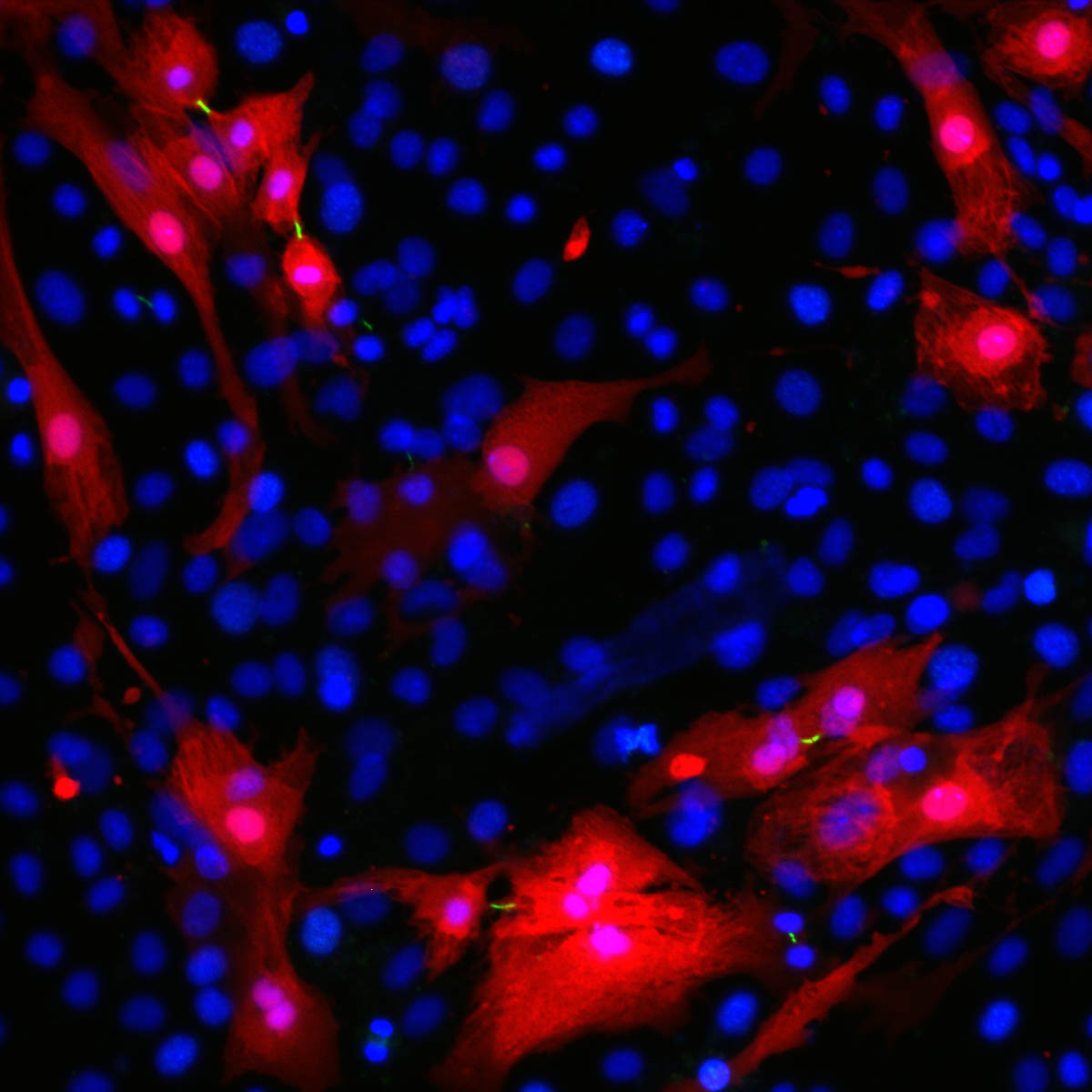
Further research revealed that after receiving the ERBB2 signals, the adult cardiomyocytes were going back to an earlier, juvenile form. These “reverted” cells were not only more prone to division but upon close examination, the scientists were surprised to note that they were less attached to their neighbors and had acquired the ability to move; they were different in shape, and they could even secrete scar-busting enzymes. These features are hallmarks of a process termed epithelial-mesenchymal-transition (EMT) familiar from the fields of embryonic development as well as cancer metastasis, but is unprecedented in the context of heart regeneration.
These cells divided and formed brand-new heart tissue
If the cells’ external makeovers were dramatic, differences in their internal and molecular architecture were no less striking. These began with alterations to the cells’ structural “backbone” – the cytoskeleton. In turn, the changes in the cytoskeleton invoked the activation of a particular cellular pathway, or chain of biochemical reactions, that mediate the effects of ERBB2. The central point of this pathway is a molecule called YAP, which is a strong promoter of growth and cell division. Adult cells, particularly heart cells that are meant to last for life, have all sorts of mechanisms in place to prevent its activation. In the engineered mice, the ERBB2 pathway was taking a circuitous route – using the modified cytoskeleton to bypass those inhibitors and vigorously activate YAP.
Further experiments with mice in which ERBB2 was overexpressed, but YAP was missing, confirmed this arrangement: YAP was needed for ERBB2 to convey its regenerative effects. After a few weeks of ERBB2 activation in the mice that had both ERBB2 and YAP, the mice’s hearts had their pumping capacity restored to a great extent.
That kind of signal, however, comes with its own caveat, since even the scar removal uses the same stratagem cancer cells use to break out of surrounding tissue. Tzahor says: “Since both ERBB2 and YAP are associated with cancer, we need to be sure we can turn this pathway off again within a short period of time. In essence, we engendered a sort of ‘mini cancer’ in the heart, and that is why the ability to control the timing of these signals was so important. When we stopped the signals, the cells matured again into adult, non-dividing cells.”
“In essence, the mechanisms of cell division and growth are very similar, whether the end result is the development of the embryo, wound repair or cancer,” says Aharonov. “They are all ‘drawn from the same deck’. Still, the ERBB2–cytoskeleton-YAP connection suggests the body shuffles the cards a bit. That is why a process can mirror cancer, on the one hand, and when applied transiently, repair hearts, on the other. Viewing heart regeneration through the prism of EMT highlights the fact that regeneration requires several intertwined events if we are to treat hearts that are on the brink of failure.”
This study demonstrates something which has long thought to be impossible, that heart failure is reversible – and that the heart cells themselves, given the right stimulus, hold the key,” adds Tzahor.
Also participating in this research were Avraham Shakked, Dr. Kfir Baruch Umansky, Dr. Or-Yam Revach, Alexander Genzelinakh, Dr. David Kain, Dr. Daria Lendengolts and Prof. Benjamin Geiger of the Molecular Cell Biology Department; Dr. Alon Savidor and Dr. Yishai Levin of the Nancy and Stephen Grand Israel National Center for Personalized Medicine; Yuka Morikawa the Texas Heart Institute, Houston, Texas; Prof. Jixin Dong of the University of Nebraska Medical Center, Omaha; and Prof. James F. Martin of Baylor College of Medicine, and the Texas Heart Institute.
Prof. Eldad Tzahor is Head of the Yad Abraham Research Center for Cancer Diagnostics and Therapy; his research is also supported by the Zuckerman STEM Leadership Program; Israel Englander; the Dr. Dvora and Haim Teitelbaum Endowment Fund; and Pearl C. Vapnek.