Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Small aquarium fish that fail to swim toward schools of fish on the side of their tanks are yielding clues to human conduct. New research in the lab of Prof. Gil Levkowitz of the Weizmann Institute of Science’s Molecular Cell Biology Department follows the effects of a single protein on individual nerve cells in the brains of transparent zebrafish – effects that translate into social behavior patterns. This study led to the identification of new neurons that act as switches between two hormones, possibly keeping the balance between feeling stressed-out and outgoing.
The development of some neurons in a part of the brain called the hypothalamus is directed by a protein called transcription factor orthopedia, or Otp. Otp is clearly crucial, as mammals lacking the gene to make this protein do not make it past birth. But Levkowitz and his group were on the trail of elusive effects connected with this protein – those that control social behavior and anxiety. Such effects may seem subtle, but malfunctions in these systems play a role in such neurodevelopmental disorders as autism. It turns out, fortuitously, that the small, transparent zebrafish have two separate Otp genes that produce nearly identical proteins – Otpa and Otpb. This means that fish bearing a mutation in either one of these Otp genes can be used to investigate its physiological effects in adult animals.
A normal fish will swim over to investigate a so-called social zone
The research was led by PhD student Einav Wircer and included Drs. Janna Blechman and Nataliya Borodovsky of Levkowitz’s group; Dr. Michael Tsoory of the Weizmann Institute’s Veterinary Resources; Prof. Rui Oliveira of the Instituto Gulbenkian de Ciência and the ISPA – Instituto Universitário, Lisbon, Portugal; and postdoctoral fellow Dr. Ana Rita Nunes, who has since moved to Portugal but is continuing to collaborate with Levkowitz’s lab.
The group observed that missing all copies of the zebrafish Otp genes was lethal, as in mice. But when just one was available, the normally social fish exhibited asocial behavior, and their anxiety response was abnormal, as well. For example, a normal fish will swim over to investigate a so-called social zone: a visible compartment in which a school of zebrafish swims to one side of their tanks. Mutant fish without the Otpa protein displayed measurably decreased tendencies to swim near the social zone. On the other hand, when faced with a new environment, fish with intact Otps will swim slowly around the middle first, and then gravitate toward the sides, as they apparently lose their initial apprehension in the unfamiliar, possibly dangerous environment, and acclimate. The mutant fish, when placed in a new tank, did not exhibit such normal “anxiety” behavior.
The researchers then showed that Otp directs the development of the neurons that produce the hormone oxytocin, which is known to affect social affiliation, trust and sexual behavior. “Oxytocin is often called the love hormone because of its social effects, but that is a misnomer. It has a number of functions in the body, including reducing stress and the modulation of appetite and metabolism.” says Levkowitz.
The scientists found that in one of the Otp mutants, a small cluster of previously unidentified neurons in the zebrafish hypothalamus was producing an increased amount of oxytocin. When they pinpointed and destroyed just these particular cells with a laser, a feat enabled by the see-through skin of the fish, the fish behaved in the same asocial manner as the mutants. Further investigation revealed that these particular neurons control the production of two different hormones – oxytocin and CRH, a hormone involved, among other things, in the response to anxiety and stress. Levkowitz says that these neurons could be a switch between the “social” and “stress” states, or they might produce both at once, functioning to regulate their relative levels.
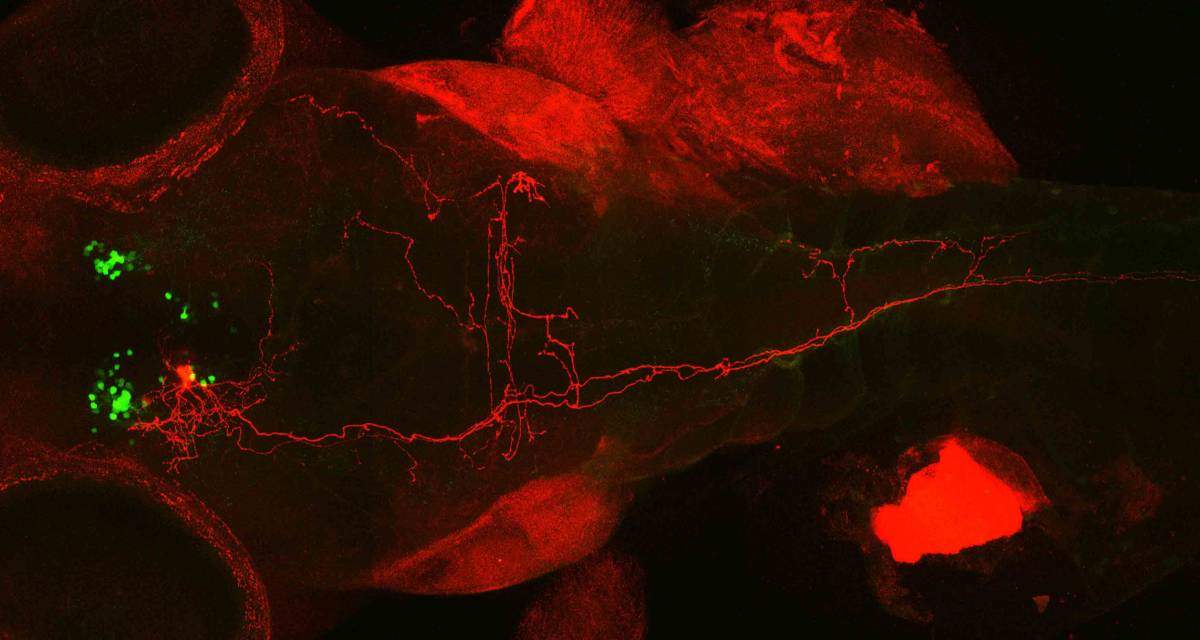
In the next step, Wircer developed a genetic method of labeling neurons with a red fluorescent protein and tracing the individual newly-discovered cells within the whole dense network of oxytocin-producing neuron extensions, these labeled with a green fluorescent protein. She then repeated the experiment for several dozen nerve cells. The images, each illuminating one cell in red among the green neuron network, revealed cells with bushy, many-branched extensions – dendrite trees – on one end and single long, hair-like extensions on the other – axons. This, says Levkowitz, is typical of neurons that take in sensory input and convert it into a signal that is relayed into the body. Some of the axons – the extensions that conduct the signals – went all the way down to the fish’s tail, suggesting that the sensory input is converted to swimming impulses.
“Our hypothalamus develops in almost exactly the same way as that of the zebrafish,” says Levkowitz, “and we almost certainly have the same ‘switching’ neurons. But in the human brain, especially the hypothalamus, which controls so many different functions, it would be close to impossible to locate them. In the zebrafish, we are starting to understand how development leads to future behavioral patterns and how a particular protein directs this process. This could help us, in the future, understand how neurodevelopmental diseases develop and progress into adulthood.”
Prof. Gil Levkowitz's research is supproted by the Adelis Foundation; and Howard and Cindy Garoon, Winnetka, IL. Prof. Levkowitz is the incumbent of the Elias Sourasky Professorial Chair.