More than half of the human body, by weight, is made up of water, yet scientists studying body chemistry have traditionally regarded water as a mere bystander – a neutral environment for various biochemical reactions. But that is starting to change: Current research is showing that water plays an active role in many bodily processes. In
a new study reported in the
Proceedings of the National Academy of Sciences (USA), researchers from the Weizmann Institute of Science, Ruhr University Bochum in Germany and Torrey Pines Institute for Molecular Studies in Florida reveal a surprising performance by water molecules – one that may open up new avenues for the faster and more effective design of drugs for cancer, autoimmune diseases and other disorders.

The study focused on a biochemical reaction that routinely occurs in the lungs, liver, skin and most other body tissues. Enzymes carry out this reaction to dismantle collagen, the main component of the scaffolding known as the extracellular matrix, which provides structural support for cells. Such enzymatic dismantling is part of ongoing “maintenance” remodeling, in which the matrix is continuously broken down and rebuilt. Using a combination of three technologies – fluorescence and X-ray, and infrared terahertz spectroscopy – the team of Weizmann Institute’s
Prof. Irit Sagi, together with teams headed by Profs. Martina Havenith and Gregg Fields, monitored the dismantling of collagen by enzymes in a laboratory dish.
In classical theory, enzymatic reactions are described by a curve: The reaction proceeds at a rate that increases at first and then levels off, continuing until the chemical broken down by the enzyme runs out. But in the new study, the scientists were amazed to discover that even after all the collagen was broken down, there were aftereffects of the reaction that persisted. Much like ripples that continue to spread after a stone is thrown in a pond, surrounding water molecules remained in motion, continuously altering their hydrogen bonds in response to the structural changes that had occurred on the surfaces of the enzyme and collagen during the reaction. The fact that these water dynamics lasted longer than the reaction itself may be an aftereffect that probably facilitates further chemical and biochemical processes in the tissue. In some of the experiments, the collagen was completely broken down within a second, whereas the water dynamics persisted for at least five times as long.
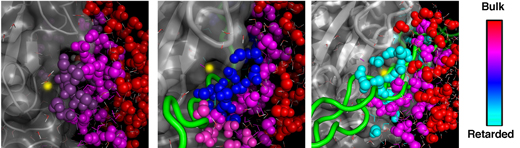
Moreover, the scientists found that the water dynamics differed depending on the type of collagen and the resulting products of the chemical reaction. This finding suggests a close connection between the water and the reaction.
This is a previously unknown biological function of water; it suggests that to obtain an in-depth understanding of enzymatic reactions in the human body, it is essential to clarify exactly how they are affected by the surrounding water molecules. Beyond clarifying the fundamentals of body chemistry, such an understanding may be crucial for the development of new drugs. For example, excessive collagen dismantling facilitates the spread of malignant cells in cancer and of inflammation-causing cells in certain autoimmune disorders. It might be possible to develop new drugs by harnessing the mechanistic insights derived from observing water-protein interactions. On a more general level, the design of a wide variety of drugs may be rendered more effective by incorporating the water-protein dynamics into computer-based drug design programs.
Prof. Sagi’s team in the Weizmann Institute’s Biological Regulation Department included Drs. Benjamin Born and Inna Solomonov. The German team, headed by Prof. Martina Havenith from the Department of Physical Chemistry, consisted of Dr. Moran Grossman, a former PhD student at the Weizmann Institute, Dr. Jessica Dielmann-Gessner and Dr. Valeria Conti Nibali. Also taking part in the study was Prof. Gregg Fields of the Torrey Pines Institute in Florida, USA.
Prof. Irit Sagi's research is supported by the Spencer Charitable Fund; the Leona M. and Harry B. Helmsley Charitable Trust; Michael and Rhoda Ambach; Cynthia Adelson, Canada; Dr. Mireille Steinberg, Canada; and the Leonard and Carol Berall Post Doctoral Fellowship. Prof. Sagi is the incumbent of the Maurizio Pontecorvo Professorial Chair.

