Much stem cell research involves embryonic stem cells – the earliest cells in the developing embryo. Embryonic stem cells are pluripotent, that is, they retain the potential to differentiate – turn into any cell type – when given the right cues. Not only does this make them invaluable for research but, theoretically, such cells could treat many problems in the body, especially diseases connected with aging as well as degenerative diseases. But because such cells are obtained solely from human embryos, their use has been extremely limited, in part due to moral issues.
That’s why the discovery in 2006 by the Japanese scientist Shinya Yamanaka that ordinary adult skin cells could be reprogrammed to become embryonic stem cells rocked the world of biological research. Though such cells are not quite identical to embryonic stem cells, they appear to share their ability to differentiate into any other type of cell. Only four genes, inserted in the cells’ DNA, were sufficient to turn back the clock, changing cells whose fate had long been “fixed” into so-called iPS (induced pluripotent stem) cells.
This finding was important, says Givol, not only because of the biomedical implications, but because it overturned the long-held assumption that cell differentiation is a one-way process that can never be reversed. Though, in practice, cells get “locked” into a specialized form, in fact, all of our tissues’ cells, from stem to skin, contain the genetic tools for creating every cell type. Reprogramming them involves, in a sense, finding out how to switch certain genes on and others off in such a way that the embryonic stem cell state is reinstated. It turns out that all four reprogramming genes belong to a small group that activate other genes in the embryonic stem cells and are afterward silenced in the differentiated cells.
But these new kinds of stem cells (iPS cells) are not ready for medical applications: The problem is the very DNA used in their reprogramming. Once inserted into the genome, the DNA remains in place and continue to function. Among other things, such cells can become cancerous if the inserted bits turn on the “wrong” genes or mutate others. In principle, any integration of DNA into the genome is dangerous and unpredictable.
Since the first iPS cells were created, scientists have been searching for ways to avoid that possibility. These have included designing intricate methods for removing the inserted DNA after it has finished the job, or else attempting to introduce the products of these genes – proteins – into cells. So far, both approaches have proved problematic.
Givol, together with his colleagues Dr. Eduard Yakubov, Prof. Shmuel Rozenblatt of Tel Aviv University and Prof. Gidi Rechavi of the Sheba Medical Center and Tel Aviv University, sought a middle way. The solution they came up with is literally a “middle man” in the cell: messenger RNA (mRNA) – the strands of genetic material copied from the DNA that carry the protein-making plans from the cell’s nucleus to its protein factories, the ribosomes. RNA, they reasoned, gets degraded after a short time, so there are no issues of foreign genetic material remaining in the cell’s DNA. More importantly, RNA does not integrate into the genome and mutations are thus avoided. On the other hand, because the proteins it encodes are produced, folded and packaged in the cells’ own machinery, they should be able to function properly in the cell.


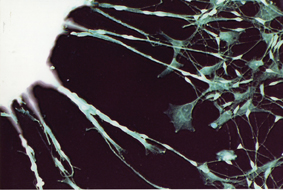
Using the four reprogramming genes, the researchers synthesized mRNA in a test tube and then inserted it into adult cells grown in culture. They repeated the procedure several times, and by the end of a week their cells were showing the expression of embryonic stem cell markers. The team observed, for instance, the activation of signature genes that are turned on only in pluripotent stem cells. And when placed on a different growth medium, the cells, which had started out as connective tissue and now were reprogrammed to be iPS cells, began to differentiate into cells with long extensions that appeared to be nerve cells.
Further experiments are needed to determine whether the new cells fulfill all of the requirements to qualify as iPS cells. But, says Givol: “We’ve proved that reprogramming with mRNA is completely possible and can be used to replace DNA for this purpose.” After publication of these results, a number of other research groups, including some that intend to develop biomedical applications, have expressed interest in the method. Givol: “RNA-induced stem (or RiPS) cells might be a way forward in the effort to develop personalized medicine. A person’s own cells could be reprogrammed, and the iPS cells could then be induced to differentiate into a specific cell type (again using mRNA). Such techniques could potentially treat a wide variety of problems, including many for which there is presently no cure.”