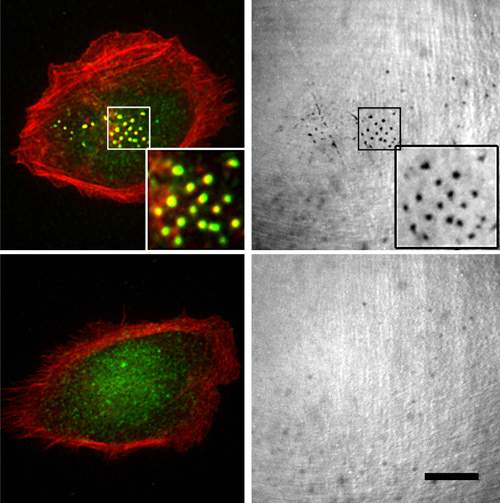
When the scientists disabled SYNJ2 in breast cancer cells in a laboratory dish using genetic engineering, the cells’ movement was impeded because they failed to form podia. The researchers then implanted mice with different types of breast cancer cells – those that had a functioning copy of the SYNJ2 gene and those that did not. The cells with the functioning SYNJ2 produced faster-growing tumors and caused more metastases to the lymph nodes and lungs than the ones without the copy.
The next step was to find a prototype for a drug that could be applicable to human patients. With the help of advanced screening technology available at the G-INCPM, the researchers sifted through tens of thousands of small molecules, ultimately identifying one that effectively blocked SYNJ2’s activity. Moreover, it worked with a great deal of precision, targeting SYNJ2 without affecting sibling enzymes, suggesting that it would cause no major unwanted side effects.
This potential therapy, under consideration for further development by Merck Serono, will be aimed at women whose breast tumors have extra copies of SYNJ2 – about four percent of all breast cancer patients. This number may not sound like much, but considering that nearly 1.7 million new breast cancer cases are diagnosed around the world each year, over time the ability to treat this particular type might translate into millions of saved lives.
Prof. Ronen Alon’s research is supported by the M.D. Moross Institute for Cancer Research; Lord David Alliance, CBE; and Mr. and Mrs. William Glied, Canada. Prof. Alon is the incumbent of the Linda Jacobs Professorial Chair in Immune and Stem Cell Research.
Prof. Tsvee Lapidot’s research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research, which he heads; the Leona M. and Harry B. Helmsley Charitable Trust; the Adelis Foundation; and the Dr. Beth Rom-Rymer Stem Cell Research Fund. Prof. Lapidot is the incumbent of the Edith Arnoff Stein Professorial Chair in Stem Cell Research.
Prof. Yosef Yarden’s research is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; the Maurice and Vivienne Wohl Biology Endowment; the Louis and Fannie Tolz Collaborative Research Project; the European Research Council; and the Marvin Tanner Laboratory for Research on Cancer. Prof. Yarden is the incumbent of the Harold and Zelda Goldenberg Professorial Chair in Molecular Cell Biology.