The myriads of microbes in our gut, collectively termed the microbiome, are considered important to our health, but they can also harbor bacteria that contribute to inflammatory bowel disease or other disorders. Currently, however, it is impossible to target such disease-contributing bacteria without harming the surrounding beneficial microbes. Antibiotics kill friendly microbes along with the harmful ones, and in any event, they tend to trigger bacterial resistance and to have side effects. In a study published today in Cell, Weizmann Institute of Science researchers have demonstrated the feasibility of a potential therapy for killing inflammation-causing gut bacteria in a targeted manner: by using viruses that infect them.
Phages, or bacteriophages, as these viruses are known, are the most abundant organisms on Earth; they are found wherever there are bacteria, including in the human gut. Attempts to enlist these viruses in treating infectious disease go back to the early 20th century, right after phages were first discovered, but this line of research was abandoned soon after the advent of antibiotics. In the new study, the Weizmann researchers recruited phages for eliminating bacteria that don’t just cause infectious disease, but also spur inflammation and gut damage, contributing to inflammatory bowel disease.

“There are thousands of different phages, and their big advantage is that each of them specializes in attacking a different type of bacteria,” explains Prof. Eran Elinav of Weizmann’s Systems Immunology Department, who headed the research team. “This enabled us to harness phages to target only those gut bacteria that contribute to disease. To our knowledge, this constitutes the first ‘silver bullet’ approach promising a precise suppression of disease-causing gut microbes, without harming the surrounding microbiome.”
The study, conducted in collaboration with Prof. Rotem Sorek of Weizmann’s Molecular Genetics Department, was led by postdoctoral fellows Drs. Sara Federici, Rafael Valdés Mas and Denise Kviatcovsky from Elinav’s lab, together with Dr. Sharon Kredo-Russo and other researchers from BiomX Inc., a clinical-stage microbiome company advancing novel phage therapies that target specific pathogenic bacteria, based on Weizmann Institute research under exclusive license from Yeda Research and Development Company Ltd., Weizmann’s technology transfer arm.
The scientists started out by identifying the exact bacterial strains that play a role in human intestinal inflammation. They compared the composition of gut microbes in healthy volunteers to that in people with two major forms of inflammatory bowel disease, ulcerative colitis and Crohn’s disease. A detailed computational analysis helped them zero in on several bacterial strains not found in the healthy individuals that were substantially enriched in people with the disease, particularly in those whose condition was worsening. The study participants were recruited from four countries in different parts of the world – France, Germany, Israel and the United States – to make sure that the results would hold true regardless of locale. After identifying several strains of the Klebsiella pneumoniae bacterium as likely contributors to intestinal inflammation, the researchers confirmed this finding by implanting these bacteria into mice used for the study of inflammatory bowel disease. Indeed, the human Klebsiella pneumoniae strains associated with this disease worsened inflammation and intestinal damage in recipient mice.
""Our vision is to eventually develop personalized therapies for a variety of disorders, in which the disease-causing strains of gut bacteria will be identified in each patient and a phage cocktail will be designed to kill only those strains"
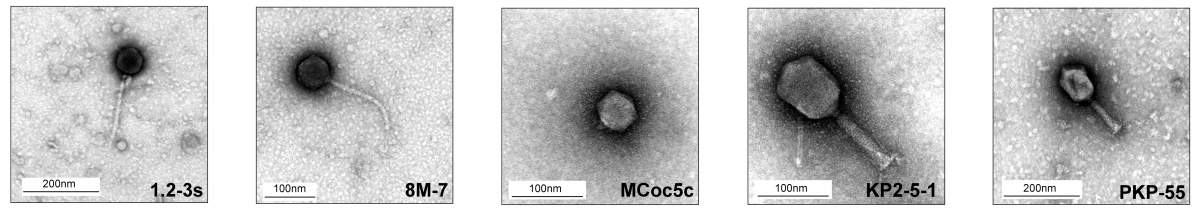
Next, the researchers screened thousands of phages, selecting about 40 that were most active against the human bacterial strains they had identified as being related to intestinal inflammation. Simply applying the phages would not be enough, however, since bacteria and phages engage in an ongoing arms race, in which the bacteria are constantly developing resistance to the phages. CRISPR, for example, a common gene editing tool, is based on a protective mechanism that bacteria employ to identify and destroy phage DNA. The Weizmann scientists used recent insights into the molecular mechanisms of this arms race in order to give their phages the upper hand against the bacteria. That is, they looked for the ideal combination of phages that would prevent the bacteria from fighting back. A cocktail of 5 phages was selected based on genetic profiles, structural features revealed through electron microscopy and extensive combinatorial screening for activity against a variety of Klebsiella pneumoniae strains, including ones resistant to antibiotics. Taken together, these 5 phages prevented the emergence of bacterial mutants that could spread resistance.
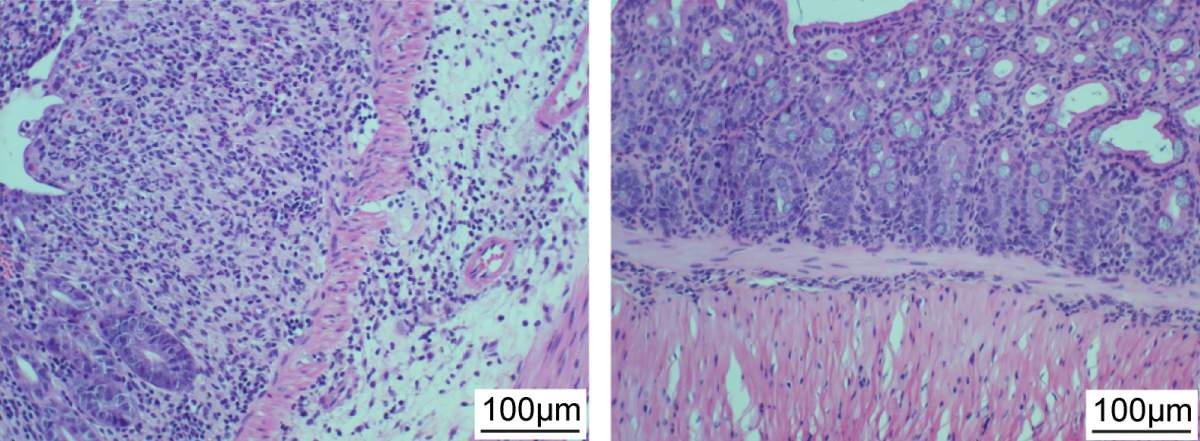
In a laboratory dish, the cocktail proved effective in killing Klebsiella pneumoniae obtained from patients with inflammatory bowel disease. In a subsequent study in mice, the cocktail significantly reduced intestinal inflammation and tissue damage caused by these bacterial strains, as well as mortality stemming from the inflammatory disease. In a laboratory system simulating the human gut, two representative phages from the cocktail were shown to be stable when used together with antacids. In a follow-up Phase I clinical trial with 18 healthy volunteers, the phages were found to be well-tolerated. Importantly, the phages persisted and even multiplied in the human intestines over time, while causing no unwanted, off-target changes in the rest of the gut microbes.
If the phage cocktail is found to be safe and effective in larger clinical trials, it may become the basis for developing therapies for not only inflammatory bowel disease but also other disorders found to be affected by gut microbes, including obesity, diabetes, neurodegenerative disease and perhaps even cancer.
“Our vision is to eventually develop personalized therapies for a variety of disorders, in which the disease-causing strains of gut bacteria will be identified in each patient and a phage cocktail will be designed to kill only those strains,” Elinav says.