Considering that all of the 100,000 or so different proteins in our bodies are made of chains constructed of thousands of linked amino acids, the error rate is quite low. Yet now and again, the wrong bit of material will make its way onto the assembly line in the protein factory – the ribosome – and end up in the polypeptide chain. Since the sequence of amino acids determines the shape of the folded protein, as well as its function, even one wrong link in the chain can spell disaster for some proteins.
Prof. Mark Safro and his colleagues Drs. Nina Moor and Liron Klipcan of the Structural Biology Department in the Faculty of Chemistry recently revealed how one of the most common substitutions can slip right by the molecular equipment that is meant to prevent mistakes. Their findings may be relevant to many disorders, including Alzheimer’s disease.
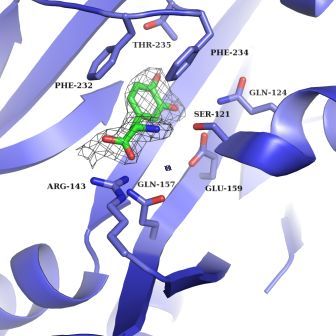
Safro and his group investigate a central step in the complex protein manufacturing process – one that helps ensure the fidelity of the translation from instruction list to finished protein. This step involves an interaction between an adaptor RNA molecule called tRNA and an enzyme known as aminoacyl-tRNA synthetase. If the tRNA can be thought of as the “trucks” that carry the individual amino acids to the assembly line in the ribosome, aminoacyl-tRNA synthetase is the “loader” that puts the amino acids on the trucks. Each such loader is familiar with one amino acid, and this is the one it captures. But some synthetases have an added responsibility. Of the 20 amino acids from which all proteins are built, some of the building blocks are easily confused with one another, and the synthetases for these components have evolved an extra function, called “proofreading,” for double-checking the shipment before sending it on its way.
Such is the case with the amino acid tyrosine, which differs from a second amino acid, phenylalanine, only by the addition of two simple atoms – an OH group. To complicate matters, tyrosine can also be mistaken for a molecule that resembles an amino acid but has a totally different function. This molecule is levodopa, or L-Dopa, better known as the common treatment for Parkinson’s disease. L-Dopa resembles tyrosine because it is made in the body by adding yet another OH group to a tyrosine molecule. This small “quasi” amino acid is able to cross into the brain, where it is converted into dopamine – the neurotransmitter that is under-produced in Parkinson’s patients. But when L-Dopa is accidentally incorporated into a protein instead of tyrosine, that second OH group becomes a problem. This tiny addition is chemically active, and it can cause the proteins to clump together into aggregates that don’t break down easily.
Safro and his team realized that the question of L-Dopa inclusion was complicated by the fact that there are two types of aminoacyl-tRNA synthetase – one in the cell’s cytoplasm and one in organelles called mitochondria, the cell’s power plants. When the researchers crystallized both types to reveal their structures, they found that the mitochondrial synthetase is a bare-bones, stripped-down version of its counterpart in the cell body. Among other things, it lacks the proofreading equipment.
Next, the team asked how capable either version is of recognizing L-Dopa and preventing it from getting into the protein chain. Using a variety of experimental methods – including a tour de force of crystallization in which they succeeded in capturing the 3-D structures of synthetase and L-Dopa acting together, as well as kinetic experiments – they showed how the mistake occurs. It appears, says Safro, that in this particular instance, the proofreading mechanism is not up to the task. The L-Dopa assumes the same orientation in the synthetase as tyrosine and thus, to the proofreader, it can look identical. While failure to recognize the false amino acid was seen in both versions, it was especially apparent in the stripped-down mitochondrial synthetases – which, unfortunately, have a greater impact on human health.
Mistakes in protein assembly are fairly rare; L-Dopa appears to be a somewhat unique case of mistaken amino acid identity. But it may also be a critical one: Protein aggregates like the ones caused by faulty L-Dopa inclusion are implicated in Alzheimer’s disease, and Safro believes that this “blind spot” in the proofreading machinery may be an important contributing factor.
Prof. Mark Safro is the incumbent of the Lee and William Abramowitz Professorial Chair of Macromolecular Biophysics.