The laws of thermodynamics – the basic laws of energy exchange in physical systems – apply to all machinery. Until now, most physicists would have said that a machine built of a single atom, though it may operate according to the strange rules of quantum mechanics, is subject to the same thermodynamic restrictions as a steam engine. But until recently, no one could actually prove this assumption. Now, research by
Prof. Gershon Kurizki in the Institute’s Faculty of Chemistry, conducted together with scientists in the Czech Republic and Poland, suggests that quantum machines may operate under somewhat different sets of rules. Although the basic laws of thermodynamics remain valid in the quantum realm, some of the restrictions they entail may differ from those encountered in large, “classical” machines. In theoretical work described in
Physical Review Letters and
Physical Review, the group revealed new facets of quantum machinery for converting heat energy to work, or work to cooling.
The starting point for the present research was a surprising effect that had previously been discovered in Kurizki’s group: Measuring (which is a form of observing) a quantum system – for example a single atom – causes it to heat up or cool down. Whether it heats or cools depends on the rate of measurement. Normally an atom placed in a larger system exchanges heat with its environment – known in scientific terms as a “heat bath.” Such a system should reach an equilibrium in which the temperature of the atom is the same as that of the heat bath. But from the point of view of the atom, energy is still being passed back and forth periodically between it and its surroundings.
In 2008,
Kurizki’s group showed, in an article that appeared in
Nature, that repeated measurements can temporarily decouple this oscillation so that increasing or lowering the temperature of the atom depends only on the rate of measurement. This means that the atom can be heated or cooled beyond the temperature of the surrounding bath by just observing it – in apparent violation of the laws previously believed to be imposed by thermodynamics. That finding was
corroborated in an experiment conducted by the Weizmann Institute’s Dr. Gonzalo Alvarez and
Prof. Lucio Frydman, which was published in 2010 in
Physical Review Letters.
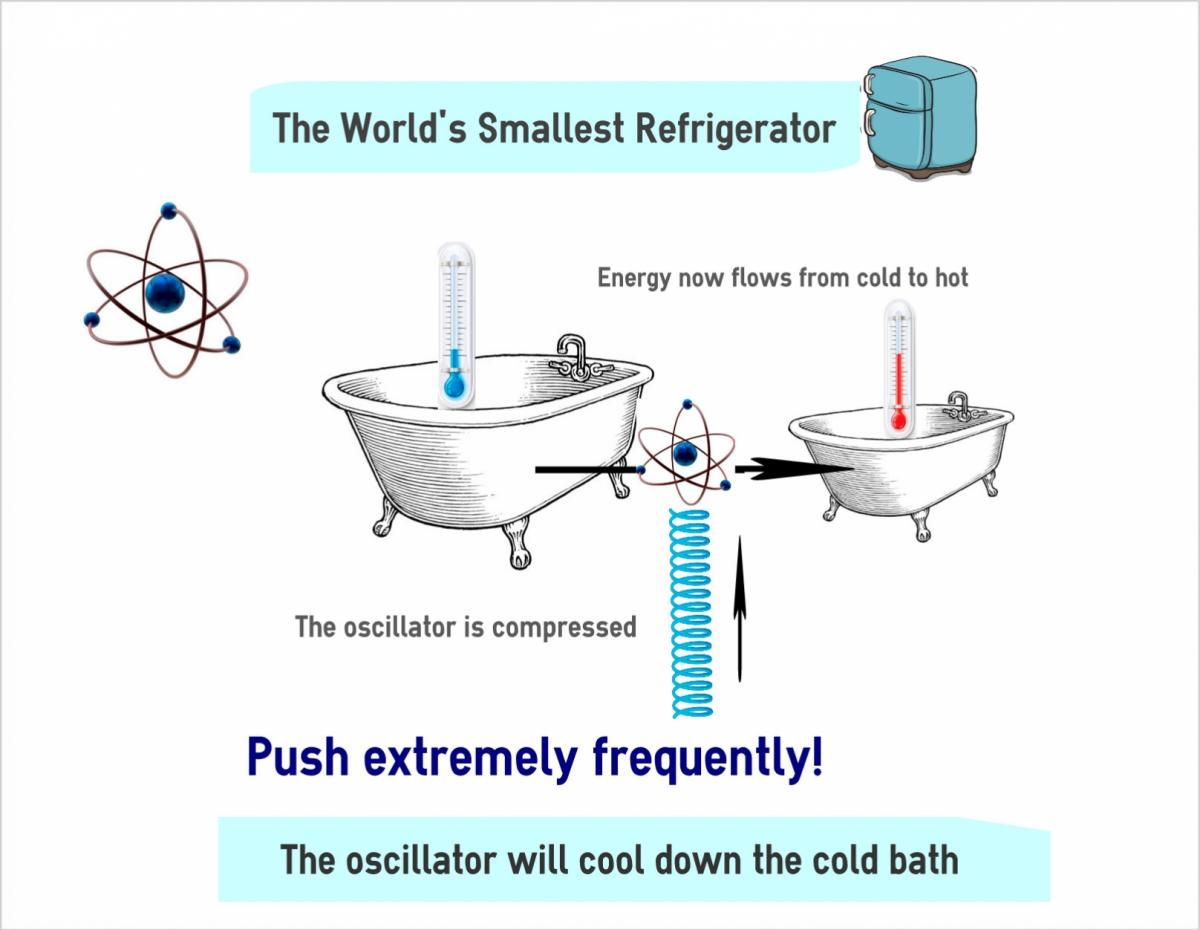
Kurizki and research student David Gelbwaser-Klimovsky have now found that the same quantum phenomenon gives the atom the ability to influence its energy exchange with the outside world in the form of useful work. If the atom, after being immersed in a bath, is then hooked up to an oscillator, and the measurements are performed on the atom at the right rate, the atom will amplify the oscillations and thereby execute work. This finding has implications for current theories – for example, the widely accepted principle proposed in 1961 by Rolf Landauer which states that work obtainable from a measurement must not exceed the energy cost of erasing its record from the observer’s memory. Here, no such record is required, so Landauer’s cost does not apply to the work extracted from the measurement.
That setup led the scientists to invent the world’s smallest refrigerator: They showed that if the atom is coupled to two baths – one heating the atom and the other cooling it – part of the heat will be converted to work that can have an amplifying effect on the oscillator. When the frequency of those oscillations gets high enough, the machine becomes a quantum refrigerator, lowering the temperature of the already-cold bath.
On a basic level, these findings may help bridge the gap between classical thermodynamics and quantum mechanics. On a more practical level, they may have implications for the cooling of tiny electronic components and nanodevices. Today, the technical difficulties in cooling such devices are one of the main limitations on chip miniaturization.
According to Kurizki, the findings may call into question one version of the third law of thermodynamics as it was first formulated by Walther Nernst in 1908. As Nernst put it, it is impossible for any procedure to lead to an absolute-zero temperature in a finite number of steps. Indeed, the classical idea of cooling involves energy or heat flowing from a warmer to a cooler substance, implying one could never actually get down to absolute zero. But the new findings suggest otherwise. Kurizki: “It may be possible to attain absolute cooling if the bath is a chain of spins (magnetized particles) connected to a single-atom ‘refrigerator.’ That chain of spins could fully freeze. Not a few physicists who have been trained to accept the third law as Gospel will be uncomfortable with this prediction. But that, ultimately, is what drives science – surprises and debate.” As for experimental proof, Kurizki thinks the theory may be tested by experiments in ultra-cold gases.
Prof. Gershon Kurizki is the incumbent of the George W. Dunne Professorial Chair of Chemical Physics.