Gur Cohen and Lapidot, together with the teams of
Prof. Irit Sagi and
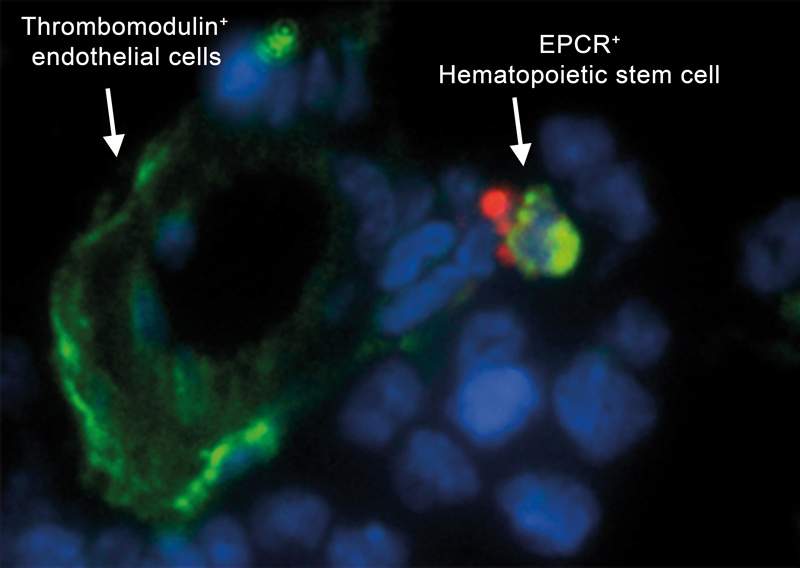
Dr. Ayelet Erez of the Weizmann Institute’s Biological Regulation Department, and Prof. Charles Esmon and Prof. Wolfram Ruf, coagulation experts from the USA and Germany, discovered that two pathways that had traditionally been viewed as coagulation- and inflammation-related actually have an independent function. They soon realized that the coagulant pathway was really two pathways – the procoagulant pathway and another, named the anticoagulant pathway. And these two pathways were active all the time, not just when there was an injury to heal. This implied that both are intrinsic to the process in which blood-producing stem cells are retained in or released from the bone marrow.

Why are there two pathways in this system? Further investigation revealed that the procoagulant pathway, though its name implies adhesiveness, is actually needed to “unstick” the stem cells from their protected niches so they can go forth into the blood stream. The anticoagulant pathway, in contrast, keeps the stem cells fixed in place in their bone marrow niches, where they are protected from damage or injury.
The body apparently needs to keep both pathways active in order to maintain a balance, replacing worn-out blood and immune cells while conserving the store of durable stem cells for future use throughout life. When the anticoagulant pathway was suppressed in mice, their supply of blood-forming stem cells in the bone marrow ran out. Further testing in mice suggested, however, that suppressing the anticoagulant pathway to coax the stem cells out of their protective niches might make these cells more sensitive to chemotherapy.
One of the team’s more surprising findings was that the blood-forming stem cells produce extremely low levels of nitrous oxide (NO), a free radical that is used throughout the body for signaling. The low NO levels are initiated by the anticoagulant pathway. This increases stem cell retention as it facilitates their adhesive interactions with the surrounding bone marrow. Procoagulant signaling, in contrast, increases NO production, so the low levels appear to be protective.
Changing cell fates
Next, the team asked whether they could use this information to affect the fate of these blood stem cells. This is especially relevant to clinical bone marrow transplantation and cancer of the blood – leukemia. The stem cell transplants that are often used to treat the disease depend on the migrating stem cells in the bloodstream homing into the bone marrow and settling in there. In this process, the anticoagulant pathway, with its low levels of NO, could be crucial for establishing a viable supply of stem cells and continued blood production. When the researchers reduced the NO levels in mice’s bone marrow, they indeed found that larger amounts of stem cells managed to repopulate in those receiving transplants.
Sometimes, however, cancerous stem cells can remain in their protective bone marrow niches, causing the cancer to return long after chemotherapy has wiped out the disease in the bloodstream. Working with mice that had inhibited anticoagulant systems, the researchers discovered a possible mechanism that limits NO levels in the bone marrow, thus enabling leukemic stem cells hiding there to escape chemotherapy. This insight may lead to new ways to remove their protection and eliminate such cancerous cells.
Completely new to scientists
Lapidot: “These two stem-cell-regulating pathways are completely new to scientists. Among other things, this research benefitted from the great synergy we have here at the Weizmann Institute – Prof. Sagi’s lab is the only one in the world to produce the molecules that block one of the pathways leading to NO generation; Dr. Erez is an expert on NO signaling; and the highly advanced equipment available in the Biological Services, with the help of Dr. Ziv Porat, enabled us to trace the actions of individual stem cells and proteins involved in the system.”
Gur Cohen: “What started out as a question about how the periphery and center of our blood system communicate after injury turned into a revelation about the way in which our blood system continues to function throughout our lives. We hope that the discoveries we made will eventually help to develop better bone marrow transplantation protocols and, hopefully, will be exploited in future studies to prevent leukemia relapse.”
Prof. Tsvee Lapidot's research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research, which he heads; the Leona M. and Harry B. Helmsley Charitable Trust; the Adelis Foundation; Pascal and Ilana Mantoux, Israel/France; and the Dr. Beth Rom-Rymer Stem Cell Research Fund. Prof. Lapidot is the incumbent of the Edith Arnoff Stein Professorial Chair in Stem Cell Research.