We know that water is essential for life. But the scientists studying life’s processes tend to ignore the water, treating it, at best, as the fluid in which everything floats. That is mainly because water molecules are extremely tiny and fast – even a single protein molecule can be thousands of times larger and slower. The microscopy methods used to observe large biological molecules are usually not able to capture the details of the thousands of water molecules around them.
To find out whether water is more than just the stuff proteins swim in, researchers recently pooled their expertise to see what happens when water molecules interact with an active enzyme.
The results, which appeared recently in
Nature Structural and Molecular Biology, show that water plays a role in at least one step in the enzymatic process, helping the enzyme to recognize the target site on a second protein.
The particular enzyme chosen belongs to a protein family that has been extensively studied in the lab of
Prof. Irit Sagi of the Weizmann Institute’s Biological Regulation Department. This enzyme and its various family members digest other biological molecules; they play a crucial role in everything from cell migration to development and tissue remodeling, and they can also enable cancer cells to migrate in the body.
Sagi, whose innovative, time-lapse, X-ray-based methods have been used to create “movies” of crucial protein activities, teamed up with the group of Prof. Martina Havenith of Ruhr University in Bochum in collaboration with Prof. Gregg Fields of the Torrey Pines Institute for Molecular Studies in Florida. The team combined Sagi’s method with terahertz spectroscopy – based on short pulses of terahertz radiation – to reveal the dynamics of the water molecules together with those of the active enzyme. This novel combination enabled them to record the data at atomic resolution and in real-time.
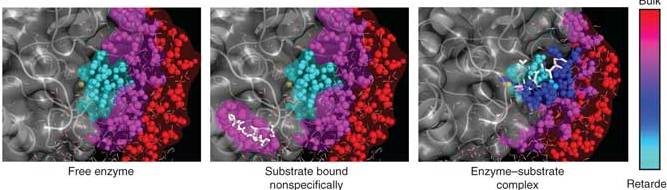
The enzyme has a metal ion (in this case, zinc) at the core of its active site. This ion, which sits in a cleft in the enzyme structure, mediates the total electric charge in the cleft during the enzyme reaction. Water is naturally drawn to such charged atoms: The oxygen side of the molecule has a slightly negative charge while the two hydrogen atoms, bound at an angle to the oxygen, give the other side a slightly positive charge. (This polarity is what keeps water liquid, as the molecules form brief bonds before sliding past one another.)
The team found that nanoscopic molecular motions of the water in the cleft were very different from those of water molecules surrounding the enzyme or located farther away in the solution. In the presence of the metal ion, the water molecules in the cleft exchanged bonds with one another very slowly. The effect of slowing the bonding was to turn the water viscous – more like thick honey than flowing liquid. In the early stages of the enzyme’s activity, the scientists observed a direct correlation between the transitions from one conformation to another and changes in the motions of the water molecules around the enzyme. As the process continued, the slow-bonding water molecules in the cleft cleared the space for the incoming protein target. The researchers believe that this change in water motion is a general phenomenon that helps enzymes bind, in the right conformation, to the proper site on the target protein substrate.
“The marriage of water to protein is really quite a complex process. By combining novel structural-biophysical tools with protein engineering, we managed to advance our understanding of nature’s designs,” says Sagi.
Does water play additional roles in the actions of this enzyme? How does it participate in other biomolecular processes? For Sagi, Havenith and their teams, this study is just the beginning. They believe that understanding the precise role of water in the actions of many different types of biological molecules may be especially useful for designing drugs, including some that they themselves are in the process of developing.
Prof. Irit Sagi’s research is supported by the Spencer Charitable Fund; the Leona M. and Harry B. Helmsley Charitable Trust; Cynthia Adelson, Canada; Mireille Steinberg, Canada; the Leonard and Carol Berall Post Doctoral Fellowship; and the Ilse Katz Institute for Material Sciences and Magnetic Resonance Research. Prof. Sagi is the incumbent of the Maurizio Pontecorvo Professorial Chair.