Reporters broadcast news about current events, trends and people "live" from the scene. Today's viewers need only tune in to this news, wherever and whenever it is taking place, to find out what's going on in the world.
Molecular biologists are faced with the much more difficult task of trying to keep abreast of events occurring within the world of an organism. In humans, for example, scientists need to keep tabs on the activities of the estimated 20,000 – 30,000 genes that code for proteins. To complicate things further, these thousands of genes can be expressed (i.e., converted into functional proteins) in many different combinations.
Gene expression, in turn, controls many events in the body: the structure and function of a cell, various processes such as blood flow and even the progression of certain diseases such as cancer.
Scientists need ways of getting an up-close look at events in which genes are controlled by the different regulatory mechanisms. To make their job easier, they employ reporter genes – genes that code for an easily detectable protein. The popular green fluorescent protein (GFP) reporter gene, for example, is widely used by scientists for this purpose. This gene "broadcasts" its reports by giving off light when it is expressed. Researchers insert the reporter DNA into a specific region of a gene they want to study, and it flashes its message back, filling them in on how this gene is regulated, where the regulation occurs and what the activity leads to in the end. But not all reporter genes are ideal for every situation. In particular, it is difficult to detect the location and intensity of fluorescent proteins in animals or people, especially when expression of the intended gene is localized deep within the body.
Alternatives have been suggested. In particular, considerable effort has been invested in developing reporter genes whose signals can be detected by magnetic resonance imaging (MRI), a non-invasive technique that is already widely used on humans and animals. Unfortunately, most of the candidate reporters proposed so far require the administration of additional substances, called reporter probes, before the MRI can detect their signals. Such substances are shut out of many cellular events – such as fetal development or those events taking place within the central nervous system, as both present barriers that the reporter probes can't cross.
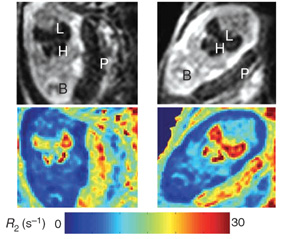
In searching for a new reporter gene that would circumvent this problem, Weizmann Institute scientists have come across a promising candidate: ferritin. According to its resume, ferritin works by chemically neutralizing iron. This protein normally minimizes iron toxicity in the cell, but when it's overexpressed, it also causes signal changes in the surrounding environment that are strong enough to be detected by MRI; no additional substrate is needed.
Prof. Michal Neeman and Dr. Batya Cohen of the Biological Regulation Department, along with research students Keren Ziv and Vicki Plaks and their colleagues, decided to give ferritin a chance to show its stuff. The scientists sent it "on assignment" by inserting the ferritin gene into a circular piece of DNA, which was in turn introduced into a special mouse strain they had developed that allows the introduced ferritin gene to be expressed in a controlled manner. They also sent along the old hand – the GFP reporter gene – which they inserted next to the ferritin gene to check independently whether it was reporting events accurately. In a further test of the method, the scientists introduced an additional gene, one that acts like a switch – it can turn both reporters either "on" or "off" simultaneously – to make sure that the signals detected actually come from the reporters themselves, and not from another source.
So far, their results, which were published in the journal Nature Medicine, show that ferritin can function as a reporter, broadcasting its reports live, via MRI detection, from the liver, endothelial cells and even during fetal development in the pregnant mouse, all without help from other materials.
Cohen: "These new results have shown that the use of ferritin as a reporter of gene expression and biological activity, especially in live animals, is not only feasible but more efficient than that of other MRI reporter genes tested so far. This approach could open many additional possibilities for studying the activation of genes during different stages of development, or detailed studies of various disease models in strains of mice bred for this purpose."
This method grew out of a joint vision that originated 10 years ago in collaboration with the late Dr. Yoav Citri.
ddf
Surfin' DNA
Any good detective knows that to solve a mystery, the evidence left at the scene is never sufficient; one needs to piece together events that took place prior to the crime. Actions that may at first appear to be irrelevant or distant may turn out to be the key to revealing the culprit. Those investigating biological mysteries also often find that clues are hidden in ostensible red herrings – seemingly tangential events that take place before the main action, for instance.
Dr. Yaakov (Koby) Levy of the Structural Biology Department investigates the "histories" of proteins, looking for activities that bear on the final outcome. How, for example, do the events a protein experiences prior to folding affect the shape it ends up with? How do proteins navigate a coil of DNA before they attach themselves to the proper binding site? Using computational models and other theoretical tools, Levy creates simplified portraits of complex biological phenomena that enable him to seek answers to these and other questions about the activities of such large molecules as proteins, DNA and RNA. While shedding light on basic biological processes, Levy hopes to help identify perpetrators that sometimes disrupt the functions of these molecules and cause problems including neurological diseases and cancer.
The life of a protein is filled with action: It folds and unfolds; phosphate and sugar groups attach themselves to its backbone and detach themselves again. The latter changes affect a protein's function: They switch it on and off, and also control the intensity of its activities. But Levy and postdoctoral fellow Dr. Dalit Sental Bechor recently showed that when sugar molecules bind to a protein, they may do more than that: They may help shape the very nature of the protein. In research that recently appeared in the Proceedings of the National Academy of Sciences (PNAS), USA, the researchers showed that the sugars that latch on to a protein directly affect its stability. They created a simple model of a protein molecule to which they attached two different types of sugar in various positions and amounts. Altogether, they produced about 60 versions of the sugar-bound protein.
Their model agreed with experimental results showing that the protein's stability rises as the number of sugar molecules increases. Further investigations of their model enabled them to explain why this happens. Like the colonel in a mystery plot who turns out to have been a double agent, the researchers found that the sugars, rather than being a stabilizing factor – as one might assume from the initial evidence – actually work to destabilize the protein, at least in one of its states. As more sugar groups bind to the unfolded protein, they increase its instability, driving it to "opt" for a stable, folded state. "By putting proteins through a range of chemical changes, nature has come up with an economical means of expanding the protein pool," says Levy. "These sugars are a control mechanism for managing the amounts of proteins in the folded and unfolded states."
Another subject Levy explores with his models concerns the interactions between proteins and DNA. Various protein molecules must bind to specific sites on the DNA for many of the cell's most crucial activities to be carried out. These activities include gene expression, repair of damaged DNA and packaging the DNA into compact structures in the cell nucleus. The binding must be both quick and accurate: Proteins typically have only a few seconds to pick the right address from millions, or even billions, of possible binding sites. How do they manage? In research that recently appeared in the Journal of Molecular Biology, Levy and research student Ohad Givati used a theoretical model to evaluate possible search methods. The molecules could, for instance, conduct an in-depth search, trawling though the DNA letter by letter to find the right code. Alternately, they might rapidly "surf" the DNA, skipping over large parts of the sequence. According to the model, the best way for proteins to conduct a DNA search is indeed by surfing. A protein should move in a spiral path around the DNA complex, skipping over about 80 percent of the encoded sequence as it goes. Levy and his team plan to continue investigating this model to try to ascertain whether the length of the DNA molecule affects the search method, whether there are differences in approach between single- and double-stranded molecules, and whether different types of proteins adopt different methods for surfing the DNA.
Dr. Yaakov Levy's research is supported by the Clore Center for Biological Physics; the Helen and Martin Kimmel Center for Molecular Design; and the Helen and Milton A. Kimmelman Center for Biomolecular Structure and Assembly. Dr. Levy is the incumbent of the Lillian and George Lyttle Career Development Chair.
Scientist and Teacher
Tel Aviv-born Dr. Koby Levy received his B.Sc. in chemistry from the Technion in 1992. After completing a Ph.D. in theoretical-computational biophysics at Tel Aviv University in 2002, Levy went on to postdoctoral research at the Theoretical Biological Physics Center at the University of California, San Diego. He joined the Weizmann Institute's Structural Biology Department in 2006. In parallel, Levy has been involved in education: As a university student, he taught high school chemistry in Haifa, and he was later appointed to the Center for Advanced Teaching at Tel Aviv University. Today, he is a member of the executive committee of "Daniel," a non-profit organization for the promotion of anthroposophic education in Israel. Levy, his wife, Rinat, and their two children, Naama (8) and Arnon (2), live in Rehovot.