Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
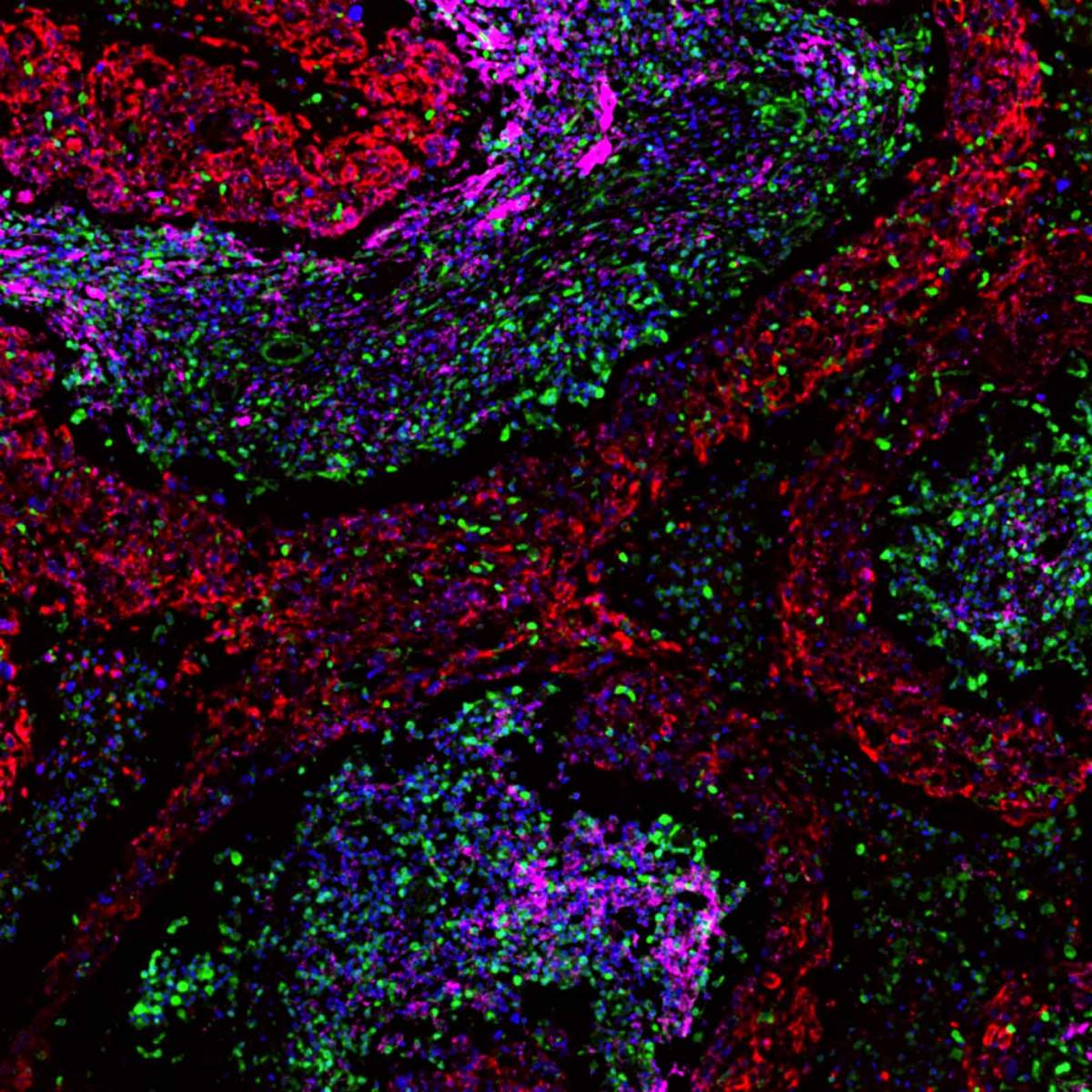
Supporting actors sometimes carry the play – not only in the theater, but in cancer too. In fact, the “help” breast cancer receives from supporting cells called cancer-associated fibroblasts is crucial for its growth. It might even be possible to predict the course of the disease from the types of fibroblasts present within a tumor. This conclusion emerges from a study by Weizmann Institute of Science researchers.
Cancer-associated fibroblasts (CAFs) are present in large amounts in breast cancer and in other malignant solid tumors; in pancreatic cancer, for example, they account for up to 90 percent of the tumor tissue. Unlike cancer cells, these fibroblasts within tumors don’t undergo mutations, but their gene expression is different from that of healthy cells, as are the roles they play. They release proteins that affect the immune response, stimulate blood vessel growth and build up the extracellular matrix that holds cells together. Dr. Ruth Scherz-Shouval of Biomolecular Sciences Department set out to learn how exactly gene expression of fibroblasts changes in malignancy, to what extent these changes are varied and dynamic, and how they affect the cells’ function.
Changes in a cell’s gene expression are reflected in its RNA. Graduate student Gil Friedman in Scherz-Shouval’s lab aimed to characterize fibroblasts from breast tumors in mice. Together with other team members and in collaboration with the lab of Prof. Ido Amit of the Immunology Department, the scientists sequenced RNA from thousands of single cells originating in tumors at different stages of growth, including metastases, and analyzed the sequencing data to understand how fibroblasts become dynamically rewired in the tumor.

The researchers found that cancer-associated fibroblasts came in several distinct subtypes, each characterized by a particular pattern of gene expression. Overall, they could be grouped into two major types, termed “p” and “s.” The ones in the p category, abbreviated as pCAFs, originated within local healthy tissue; those in the s category, or sCAFs, originated elsewhere, probably in the bone marrow.
In pancreatic cancer, they account for up to 90 percent of the tumor tissue
By isolating these two types of fibroblasts from mouse tumors and studying them in tissue culture, the scientists found a division of labor between them: The differences in gene expression translated into different supporting roles they played in malignancy. Moreover, both the gene expression and the role of these cells was dynamic – it changed as the tumors progressed. For example, in early stages of tumor growth, one subtype of the pCAFs mainly suppressed the activation of immune T cells, but in later stages, the main function of the same cell subtype was to release collagen for the extracellular matrix.
To test the relevance of these findings to humans, the scientists analyzed tissue samples collected at the Chaim Sheba Medical Center in Tel Hashomer, Israel, and at Cancer Research UK in Cambridge, from hundreds of breast cancer patients. The researchers performed a procedure that is well suited to a hospital setting: staining tissue samples with markers for different cell subtypes, based on those they had identified in mouse cells. The staining revealed that similarly to fibroblasts in mouse tumors, the ones in human tumors also fall into two major types, pCAFs and sCAFs. Furthermore, the ratio between the two types of cells correlated with disease prognosis: Survival was markedly better in patients who had more sCAFs than pCAFs. The latter finding was most pronounced in patients with triple-negative cancer and BRCA mutations, one of the most aggressive types of breast cancer that is particularly prevalent in Ashkenazi Jews.
In addition to providing a means of assessing breast cancer prognosis, the study’s findings open up new directions in the search for potential future therapies or in improving existing ones. Further studies will clarify how exactly the fibroblasts affect the microenvironment of the tumor, and this knowledge can help develop new drugs. For example, activity of fibroblasts that release signals to alter the immune response may be blocked by medications so as to allow immune cells, including those induced by various immunotherapies, to kill the tumors.
Study participants included Oshrat Levi-Galibov, Coral Halperin, Dr. Meirav Pevsner-Fischer, Hagar Lavon, Shimrit Mayer, Dr. Reinat Nevo and Yaniv Stein of the Biomolecular Sciences Department; Eyal David and Drs. Chamutal Bornstein and Amir Giladi of the Immunology Department; Dr. Avi Mayo and Prof. Uri Alon of the Molecular Cell Biology Department; Drs. Maya Dadiani, Nora Balint-Lahat, Iris Barshack and Einav Nili-Gal-Yam of Chaim Sheba Medical Center; and Drs. H. Raza Ali and Carlos Caldas of Cancer Research UK.
Dr. Ruth Scherz-Shouval's research is supported by the Moross Integrated Cancer Center; the Laura Gurwin Flug Family Fund; the Peter and Patricia Gruber Awards; the Rising Tide Foundation; the Elsie and Marvin Dekelboum Family Foundation; and the estate of Aliza Yemini. Dr. Scherz-Shouval is the incumbent of the Ernst and Kaethe Ascher Career Development Chair in Life Sciences.