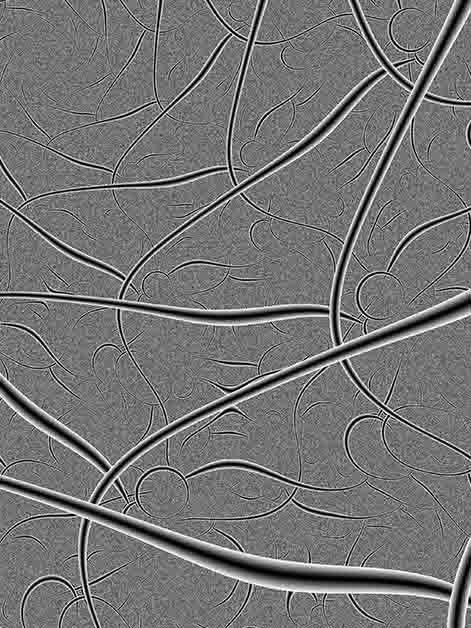
Like treetops reaching high into the sky to sense the sunlight, our sensory neurons – whose role is to collect information about what is happening in and around the body – grow long, intricate extensions known as axons. These extensions spread out throughout the body, conveying various sensations in response to different stimuli. But who is the constant gardener making sure that these extensions don’t grow wild over time? In research published in Cell Reports, Prof. Avraham Yaron and his team in the Biomolecular Sciences and Molecular Neuroscience Departments at the Weizmann Institute of Science discovered a regulatory protein that is responsible for grooming the nerve endings. The study’s findings, which shed light on the mechanisms that regulate our pain sensitivity, could help pave the way toward the development of new methods for chronic pain management.
The cell bodies of sensory neurons are planted alongside the spine and, in order to do their job properly, each of them grows an axon that divides into two when it is created: One branch grows in the direction of the central nervous system, while the other extends to various parts of the body. These axons can be incredibly long; the longest of them stretches from the base of the spine to the toes. When they reach the outer layers of the skin, they split even further into intricate “treetops” that monitor heat, pain, touch and other stimuli.

In a 2013 study, Yaron’s research group discovered that one of the cell skeleton’s regulatory proteins, known as Kif2a, is needed to prune the axons during the development of the nervous system in mouse embryos, and that the absence of this protein creates an excess of axons in the embryonic skin tissue. In the new study, a team led by research student Swagata Dey examined what happens in adult mice. The researchers first addressed a major challenge: Mice cannot survive without the gene that encodes this regulatory protein, so the scientists had to genetically engineer a mouse in which the Kif2a gene is silenced only in the sensory neurons.
Using these genetically engineered mice, the researchers discovered that the Kif2a protein continues to act as a gardener even after birth, and they showed that its absence leads to the growth of “weeds”: Each parent axon split into more daughter branches. The researchers identified a slight increase in the density of the axons in the skin of month-old mice that lacked the Kif2a-encoding gene; after three months, the situation deteriorated. The scientists concluded that the protein’s activity plays an important role in sensory neurons over the course of a lifetime and that the consequences of the protein’s absence become ever more evident with age.
""What we’ve discovered is a kind of ‘exposure therapy,’ whereby prolonged exposure to pain leads to desensitization to the pain-causing stimulus"
But does the absence of the protein affect sensitivity to stimuli and pain? “In the first month after birth, the mice revealed no hypersensitivity to stimuli in the various experiments we conducted, despite the minor increase in the density of sensory axons in their skin,” Yaron explains. “However, after three months they did exhibit hypersensitivity to pain and heat, and the intensity of their response to these stimuli increased, as did the length of this response, whereas sensitivity to touch remained unchanged.”
To examine whether this hypersensitivity to pain was connected to the structural change in the axon endings, Dey and colleagues joined forces with Hebrew University of Jerusalem researchers – Prof. Alexander Binshtok and Dr. Omer Barkai, a research student in his lab – who developed a computer model mimicking the relationships between structural changes and nerve activity. The model suggested that changes in the structure of the axon endings in the mutant mice could explain both the more intense response to stimuli and the extended time of that response.

Pain now, relief later
To validate their findings, the researchers genetically engineered mice in which the regulatory protein was absent only in those sensory neurons that express a receptor known to be involved in sensing pain: the receptor for capsaicin, the same compound that gives chili peppers their heat. When these neurons were activated, the mice exhibited hypersensitivity and behaved in a way that indicated a heightened level of pain.
The most surprising finding, however, came six months after birth: Although the density of the axon endings remained high, hypersensitivity to pain disappeared. “Most of the researchers we consulted didn’t understand why we were examining the mice again at six months,” says Yaron. “In the end, however, this repeat examination revealed that, over time, the body activates a clever compensatory mechanism, designed to rein in the overexuberant axon endings in the skin by reducing their sensitivity.”
To understand how this compensatory mechanism works, the researchers sequenced messenger RNA molecules from the sensory neurons of mice at different ages and mapped the changes in the expression levels of various genes. They discovered that when the mice reached six months of age, there was a drop in the expression of several proteins that play key roles in relaying pain sensation. Using the computer model, they showed that these changes in the levels of expression are enough to compensate for the hypersensitivity caused by the excess of axon endings.