Allowing broken furniture and appliances to accumulate can cause stress: If we fail to haul them away, our house at some point becomes unlivable. For the living cell, with its limited resources, it is critical not just to get rid of the old junk, but to recycle it for reuse. Fortunately, says
Prof. Zvulun Elazar of the Biological Chemistry Department, researchers are now realizing the importance of a kind of cellular recycling activity known as autophagy (“self-eating” in Greek). This process is vital for everything from growth and development to cancer prevention; malfunctions in the autophagy equipment can contribute to Parkinson’s and Crohn’s, as well as a number of other diseases.
Autophagy comes into play when resources are scarce, recycling less crucial components to keep the cell’s main functions going; but it is also important for general maintenance. The components are broken down in a special compartment called the autophagosome, which is enclosed in a double membrane.
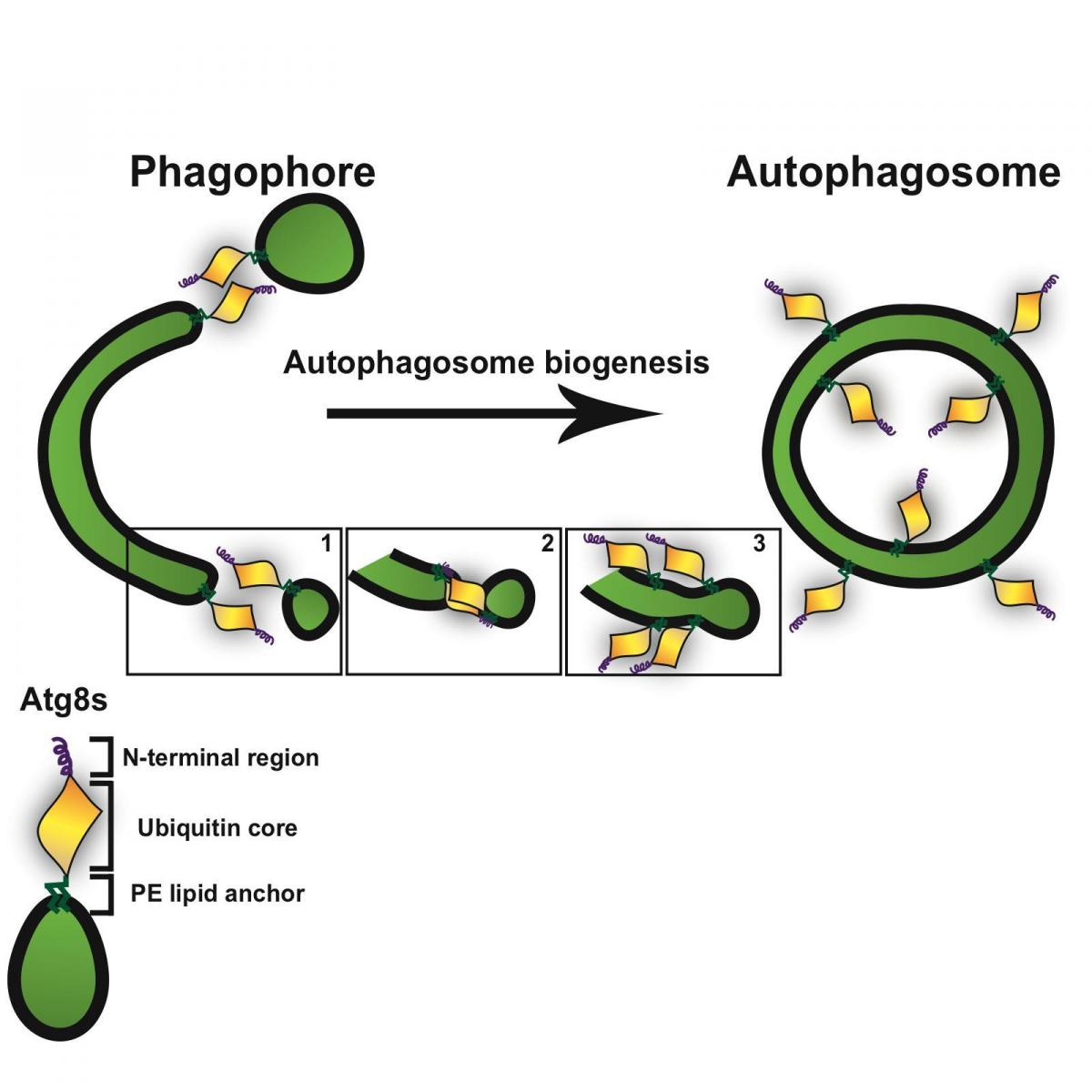
In the cell, not even the recycling equipment is left to gather dust: The autophagosome gets constructed anew each time as needed, on the spot. A unique membrane called a phagophore is assembled from bits and pieces of other membranes. As it elongates, the membrane takes on a cup-like shape that gradually extends around the cellular material slated for recycling until the compartment is sealed off completely from the rest of the cell. Elazar and research students Hilla Weidberg and Tomer Shpilka have been investigating the nuts and bolts of autophagosome assembly. In recent research published
in the EMBO Journal and
Developmental Cell, they revealed the actions of two proteins that fuse together the pieces of the autophagosome membrane.
The two proteins, known as LC3
B and GATE-16 (the latter originally identified by Elazar’s group several years ago), share some characteristics with other cellular proteins – particularly ubiquitin, a small tag that the cell applies to certain proteins to label them. Even though LC3
B and GATE-16 are similar in design to ubiquitin, they attach to lipids – the fatty molecules that constitute cellular membranes – rather than to proteins; and the bonds they form are unusually stable. Elazar and his team found that both of these proteins are necessary for constructing an autophagosome. When they blocked either one, the assembly process was incomplete.
After latching on to the phagophore, small active sites on the proteins glue the membranes together. These membranes fuse into one double-walled structure as components continue to be added to the autophagosome wall. The researchers found that the active sites on LC3B and GATE-16 each use a different mode of action, possibly explaining why both are necessary. They think that one of the proteins contributes to the elongation of the phagophore while the other may also function as the “latch” that seals the membrane shut when it reaches the proper size.
“This is the first time,” says Elazar, “that this type of membrane fusion mechanism has been demonstrated in a mammalian cell. Our appreciation of the role that autophagy plays in nearly every biological process grows stronger the more we study it. That is why it is so important to understand exactly how the equipment works.”
Prof. Zvulun Elazar's research is supported by the Louis Brause Philanthropic Fund; and the Yeda-Sela Center for Basic Research. Prof. Elazar is the incumbent of the Harold L. Korda Professorial Chair of Biology.