Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Our body’s cells have small “pairs of scissors” on the loose. These tiny molecular machines float in the cell’s cytoplasm chopping up unwanted proteins; Weizmann Institute scientists have discovered a regulatory mechanism that controls these molecular “scissors.” Their findings, reported in Nature Communications, may help develop anti-cancer drugs with reduced side effects and point toward new avenues of research into Parkinson’s disease.
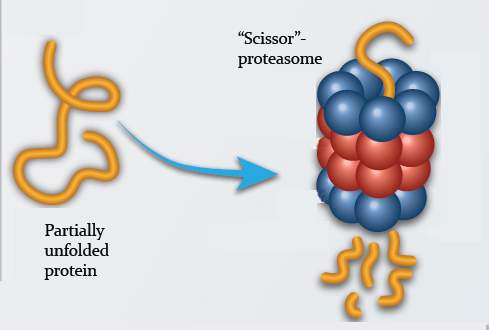
The job of breaking down unwanted proteins – ones that are worn, damaged or simply no longer needed – is so essential for the proper functioning of the organism that two different processes have evolved for accomplishing this task. In the first process, which has been more extensively studied and is better understood, a large machine-like complex called the proteasome recycles proteins that have first been labeled for destruction. The second process, discovered more recently, is performed by the molecular scissors: a proteasome molecule pared down to its essentials until it consists of nothing but the actual cutting machinery. This “scissor proteasome” doesn’t wait for unwanted proteins to be labeled; rather, it lashes out against all proteins that have lost their neatly folded, three-dimensional structure and cuts them up.
But having a pair of rampaging scissors cutting up all unfolded proteins in the cell could be problematic. On the one hand, unfolding can be a sign of damage, so it makes sense for the cell to have a way of eliminating such defective proteins quickly, without wasting time on labeling them. In fact, the scissor proteasome most commonly operates in the emergency mode known as oxidative stress, which is known to cause widespread damage to protein structure. However, nearly half of all the perfectly normal proteins in the body have been found to contain segments that are entirely unfolded. This is the case, for example, for p53, a major tumor suppressor – that is, a protein that acts as one of the cell’s anti-cancer protectors. In fact, apart from p53, the group of partially unfolded proteins includes many that regulate cell division and perform additional critical functions in the cell. So mechanisms to reign in the scissor proteasome are crucially needed.
One such control mechanism has now been identified by Dr. Michal Sharon and her team in the Weizmann Institute’s Biological Chemistry Department: Dr. Oren Moscovitz, Dr. Gili Ben-Nissan, Irit Fainer, Dan Pollack and Limor Mizrachi. They have found that a protein called DJ-1 blocks the activity of the scissor proteasome, rescuing unfolded proteins from degradation. In the study, DJ-1 was shown, for instance, to prevent the scissor proteasome from cutting up the p53 protein. The scientists also showed that DJ-1 physically binds to the scissor proteasome, which further supports its control function.
Mechanisms to reign in the scissor proteasome are crucially needed
This in-depth understanding of these protein degradation mechanisms may in the future help manipulate the system so as to make protein breakdown more selective. For example, a number of new-generation cancer drugs have been designed to prevent proteasomes from breaking down anti-cancer proteins. But these drugs also prevent the degradation of numerous other proteins, leading to side effects. By clarifying the protein degradation process in greater detail, the Weizmann study may in the future facilitate the design of drugs that will interfere with this process with greater precision.
Furthermore, the study may shed new light on Parkinson’s disease. Recent research had shown that mutations in the DJ-1 gene cause a hereditary form of this disease that starts early in life. By proposing a new role for DJ-1, the Weizmann Institute study opens up a possible new line of investigations into the underlying mechanisms of this disease.
Dr. Michal Sharon’s research is supported by the Benoziyo Fund for the Advancement of Science; the Abramson Family Center for Young Scientists; the European Research Council; the Abisch Frenkel Foundation for the Promotion of Life Sciences; and the Sergio Lombroso Award for Cancer Research. Dr. Sharon is the incumbent of the Elaine Blond Career Development Chair in Perpetuity.