Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
A pure democracy is a society consisting of a small number of citizens, who assemble and administer the government in person. James Madison
A new study at the Weizmann Institute of Science suggests that the nerve networks in our brain may function democratically; the behavior of the network as a whole is fashioned from the collective behavior of its individual “citizens.” In a unique experimental setup in which a small “society” of neurons was grown in a lab dish, the researchers discovered that each neuron, when left alone, marches to its own drumbeat. But when conditions require neurons to work together, their rhythms join together, becoming a coordinated, synchronized signal.

“One of the outstanding questions about the brain’s networks has been how their activity is controlled,” says Prof. Menahem Segal of the Institute’s Neurobiology Department. “Many have posited the existence of special ‘leader cells’ that set the rest in motion, but such cells have not been seen in action.” The neuroscientists, Segal, and postdoctoral fellow Dr. Yaron Penn, along with Prof. Elisha Moses who is a physicist in the Institute’s Physics of Complex Systems Department, investigated this question with an artificial neuron network that the Moses’s lab has been developing over the past several years.
The experimental neuron network they created is a pared-down, working model of a brain network. It contains a small number of embryonic rat neurons grown together in lab conditions that enable the neurons to communicate. This system enables the researchers to observe the neurons’ activities at close range, individually and collectively, for long periods of time.
In the experiment, the researchers varied the levels of different substances in the neurons’ surroundings; these blocked or enhanced the connections in the network. Their first surprise, says Penn, was that most of the neurons exhibited electrical activity even when they were disconnected from the network. This activity took the form of oscillations – periodic peaks and dips – and each neuron oscillated at its own pace.
Their second surprise was that when conditions were right for network activity, the signals that arose were also oscillations, and frequencies of these oscillations were close to the average of all the individual ones. This suggested a pattern of activity in which the individual neurons become increasingly connected and adjust their frequencies to that of their neighbors, until the pattern evens out. “The patterns we see in our experiments”, says Moses, “suggest that many neurons are constantly in a state in which the signal arises, democratically, from connectivity between them.”
We were able to control these two parameters – excitability and connectivity – like dials on a radio
Moses points out that many systems in nature, especially neural ones, involve oscillation and synchronization. “A cloud of fireflies flashing in tandem is an example of a collective, synchronized, oscillating system. We think that oscillation is the default mode for many neurons; this insight can help us to work out how their networks form,” he says.
The researchers further discovered exactly how the neurons regulate their network activity: It comes down to calcium ions. Letting calcium out of the body of the cell increases its excitability, letting it in increases connectivity. “We were able to control these two parameters – excitability and connectivity – like dials on a radio,” says Penn, “and thus to obtain different outputs.”
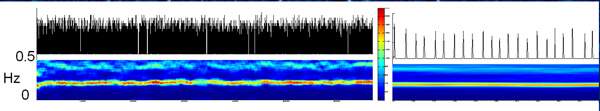
By manipulating the “dials” in the experimental nerve network, the researchers found two different mechanisms for its activation. One was the collective average, which produced a relatively slow, gentle oscillation, over a period of minutes. The other, the scientists describe as a “scream”: a sharp peak in which the electrical activity of all the neurons is highly synchronized.
Slow wave or scream: both arose from the levels of connections between the neurons, which adapted the frequencies of their oscillations accordingly. Thus, rather than a hierarchical network in which each nerve cell fires on command, the brain’s network activity may actually be something like Madison’s pure democracy: a collective, leaderless effort in which each neuron plays a role and is affected, in turn, by the group action.
“The experimental system we have developed and the result that Penn has now found,” says Segal, “is the product of a unique collaboration between a neurobiologist and a physicist – and the insights we have gained can lead us in many new directions. For example, we can ask how embryonic neurons begin to form networks, or what conditions in the brain invoke a particular network action. On the other hand, we know that such diseases as epilepsy involve an imbalance between excitability and connectivity, and this system could provide new ways to investigate what goes wrong.”
Prof. Elisha Moses's research is supported by the Clore Center for Biological Physics, which he heads; the Benoziyo Fund for the Advancement of Science; and the Lulu P. and David J. Levidow Fund for Alzheimers Diseases and Neuroscience Research.
Prof. Menahem Segal's reseach is supported by the Irving B. Harris Fund for New Directions in Brain Research.