Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Dr. Ayelet Erez decided to focus on the brain in her new study because of a puzzling aspect of ASL deficiency – a rare disorder affecting one of the enzymes that act in the liver to convert excess ammonia into a nontoxic substance secreted in the urine. Erez and other physicians had observed that patients with ASL deficiency suffer from high levels of cognitive impairment. When children with this deficiency are diagnosed early and treated immediately – including with extreme interventions such as a liver transplant – symptoms relating to liver dysfunction and abnormal levels of ammonia disappear, but strangely enough, these children still have a tendency to experience seizures and delayed cognitive development.
This suggested to Erez that apart from ASL's known function in the liver, this enzyme might play an additional role in the brain. She reasoned that clarifying the link between ASL and brain function could help control the effects of ASL deficiency in these children and this might even shed light on the causes of other types of developmental delays.
In fact, Erez’s earlier work had already supplied a clue that ASL might affect the brain via nitric oxide ‒ NO. During her postdoctoral research, when she had first discovered the importance of ASL for the manufacture of NO, she had given NO supplements to an ASL-deficient child as a treatment for his high blood pressure. Surprisingly, not only did this treatment bring his blood pressure down to normal, it also benefited the child’s mental ability: His attention span and verbal memory improved, as did his chess game.

In the new study, published recently in Cell Reports, Shaul Lerner, a doctoral student in Erez's lab in the Weizmann Institute's Biological Regulation Department, together with other researchers, wanted to determine whether ASL participates in the regulation of cognitive brain functions via NO.
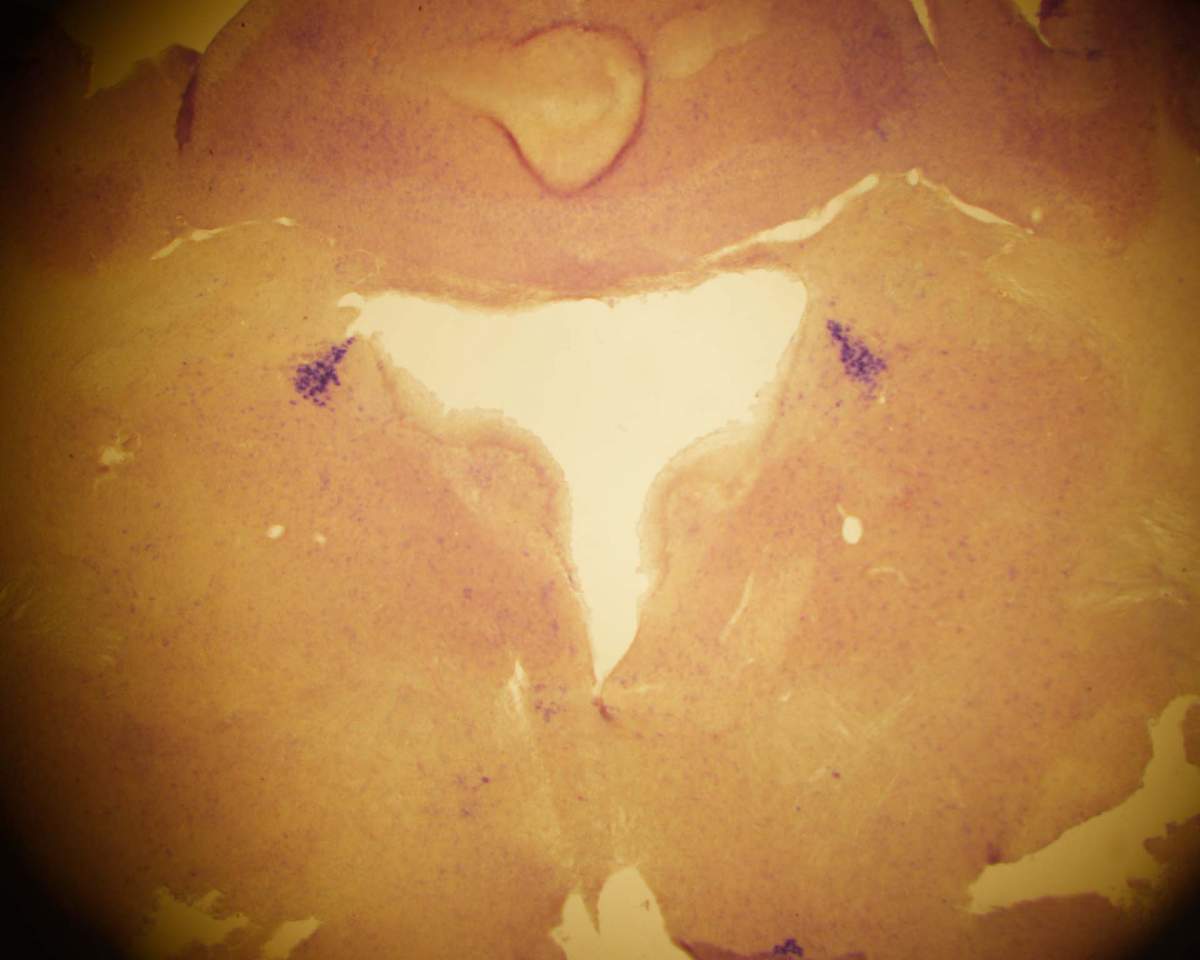
The scientists began by mapping the distribution of ASL in the brain. They found that the major site of its expression was in an area of the brainstem called the locus coeruleus (Latin for “blue place”), which is involved in regulating such attention-related functions as learning and memory, as well as the response to stress and various other physiological processes. This region serves as the central source of the essential brain chemical norepinephrine, also known as noradrenaline, the source of adrenaline.
Next, the researchers created transgenic mice in which the ASL protein was not expressed in the locus coeruleus and compared gene expression and protein changes in these and regular mice, focusing on the locus coeruleus cells. The analysis revealed that a lack of ASL reduces the levels of NO in the locus coeruleus, which, in turn, reduces the levels and activity of another enzyme, called TH for short. TH is needed for the production of norepinephrine in the locus coeruleus, so a shortage of it results in a shortage of norepinephrine, and this can potentially contribute to multiple cognitive, physiological and behavioral problems, including hyperactivity and attention deficit disorder.
To confirm that this chain of events indeed constitutes the link between ASL deficiency and problems in brain function, Erez’s research team performed a series of experiments in collaboration with neuroscientists Profs. Menachem Segal and Alon Chen of Weizmann’s Neurobiology Department. The team showed that their transgenic mice exhibited an abnormal response to stress on physiological and behavioral parameters and were prone to seizures. In fact, the symptoms observed in the mice were quite similar to the ones typical of people with ASL deficiency and those with disruptions in the function of the locus coeruleus. Remarkably, these symptoms were relieved when the mice were given NO as a supplement.
“Cognitive impairment may be caused by multiple genetic changes,” Erez says. “Rare disorders provide us with means for distinguishing the consequences of specific genetic alterations from the multiple changes that accompany the more complex diseases.”
Remarkably, these symptoms were relieved when the mice were given NO as a supplement
Adds Lerner: “Better understanding the effects of ASL on norepinephrine synthesis and on brain function, might in the future give us new tools for regulating the central production of norepinephrine in the brain.”
In the meantime, Erez, a medical doctor specializing in pediatric genetics, says that before conducting clinical trials it would be too early to conclude that NO supplements can solve such problems as hyperactivity or attention deficit disorder. “Instead, I tell parents of children with these disorders to try including in their children’s diet certain foods that are known to be rich in NO substrates, for example, spinach,” she says.
Also taking part in this study were Dr. Elmira Anderzhanova of University Hospital Bonn; Dr. Sima Verbitsky of Weizmann’s Neurobiology Department; Dr. Raya Eilam, Dr. Yael Kuperman, Dr. Michael Tsoory and Dr. Yuri Kuznetsov of Weizmann’s Veterinary Resources Department; Dr. Alexander Brandis and Tevie Mehlman of Weizmann’s Life Sciences Core Facilities Department; Dr. Ram Mazkereth of Tel Aviv University; Dr. Robert McCarter of George Washington University; and Prof. Sandesh Nagamani of the Baylor College of Medicine.
Dr. Ayelet Erez's research is supported by the Moross Integrated Cancer Center; the Sagol Institute for Longevity Research; the Adelis Foundation; the Rising Tide Foundation; Robin Lynn and Lawrence S. Blumberg; Manya and Adolph Zarovinsky; and the European Research Council. Dr. Erez is the incumbent of the Leah Omenn Career Development Chair.