Bullying, unfortunately, can be contagious. This applies not only at school or on the playground but also in the cellular neighborhood. That’s why in a new study, a team of researchers headed by Dr. Ruth Scherz-Shouval of the Weizmann Institute of Science focused not only on the cancer cells’ “bullying” behavior but also on its deleterious effects on the cells in the tumor’s surrounding microenvironment. These nonmalignant cells can help the body fight cancer, but sometimes they actually undermine this fight, becoming collaborators of the cancer cells. In the study, the scientists reveal how mutations in the BRCA genes, notorious for their role in breast and ovarian cancer, adversely affect a major subset of cells in the microenvironment of pancreatic cancer, impairing the body’s anticancer immune response.
The BRCA genes, in their normal form, play a significant role in cellular mechanisms that repair damaged DNA, but some people are born with a BRCA gene containing small changes (mutations) that disrupt its function. Although these mutations – particularly common among Ashkenazi Jews – are most known to women undergoing preventive screening for breast and ovarian cancer, they have been shown to increase the risk of cancer in men also, including tumors of the pancreas and prostate. Yet, awareness of this risk and the testing rate for BRCA mutations remain low among men.

The researchers conducted the new study on pancreatic ductal adenocarcinoma, a common and particularly aggressive type of pancreatic cancer that is still largely incurable. Only 10 percent of those diagnosed with it survive more than five years after diagnosis. Previous studies had shown that in this malignancy, cancer cells succeed in rewiring and even changing the structure and function of certain cells, called fibroblasts, in their microenvironment. Fibroblasts, which form the scaffolding holding cells in place, are basic components of every organ in our body. In pancreatic cancer, they can account for up to 90 percent of the tumor tissue. Once these cells cross over to the cancer’s side, they are reprogrammed into different subpopulations of cancer-associated fibroblasts. However, it is still largely unknown whether different cancer-related mutations, such as those in the BRCA genes, lead to different types of fibroblast reprogramming.
In an attempt to find the answer, researchers – led by Drs. Lee Shaashua, Aviad Ben-Shmuel and Meirav Pevsner-Fischer from Scherz-Shouval’s group in Weizmann’s Biomolecular Sciences Department – sought to establish whether BRCA mutations produce a unique negative effect on the fibroblasts in the tumor microenvironment of pancreatic cancer. In collaboration with Memorial Sloan Kettering Cancer Center in New York, they used innovative research methods to map and compare fibroblasts in pancreatic tumor samples containing cancerous cells with and without BRCA mutations.
Although these mutations are most known to women undergoing preventive screening for breast and ovarian cancer, they have been shown to increase the risk of cancer in men also
The scientists found that these two types of tumors had significant differences in the composition of their fibroblast subpopulations. In particular, they discovered that pancreatic tumors with BRCA mutations contain a relatively large subpopulation of certain fibroblasts – those containing the protein clusterin. This chaperone protein, which helps other cellular proteins function properly, had been found in past studies to contribute to the development of pancreatic cancer tumors.
Next, using cell culture and mouse models of cancer, the researchers silenced a BRCA gene in cancer cells, so as to mimic the mutation-induced loss of its function. These experiments revealed how BRCA mutations trigger a change in adjacent fibroblasts’ composition, even though the fibroblasts do not themselves have the mutation. It turned out that the culprit, responsible for the increased proportion of clusterin-expressing fibroblasts, is the HSF1 protein. In healthy cells this protein is activated in response to stress; in previous studies, including those conducted in Scherz-Shouval’s lab, it had been found to play a role in turning fibroblasts into cancer-assisting cells.
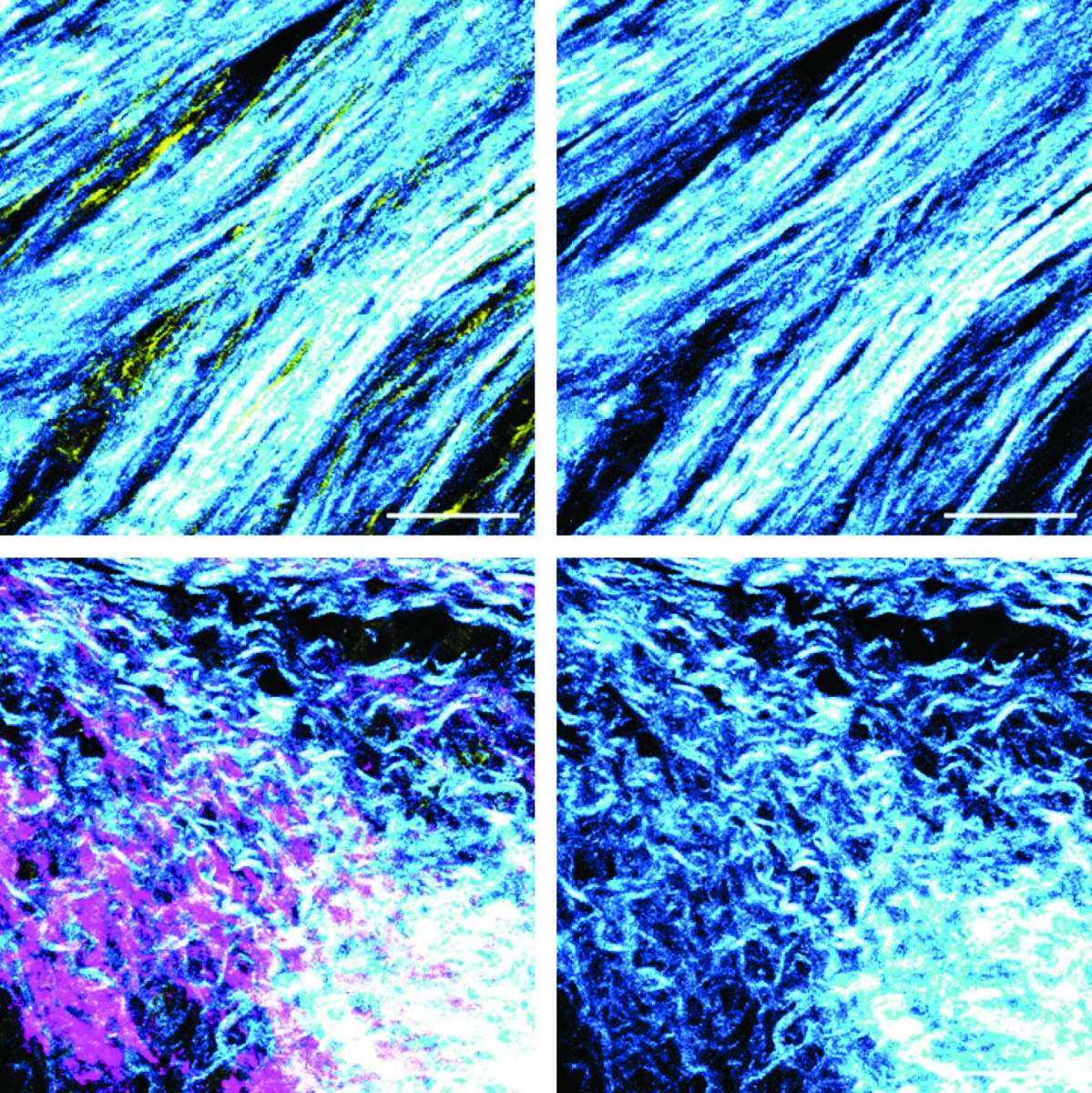
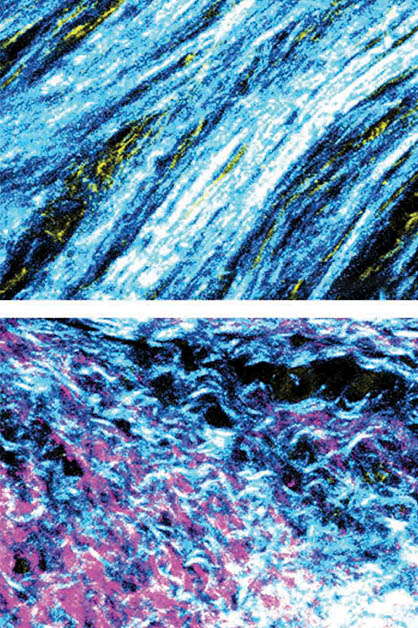
In further experiments, the researchers discovered that BRCA silencing in cancer cells induced a shift in the function of adjacent fibroblasts, causing them to start suppressing the immune system’s T cells. The researchers also identified a structural change in the fibers produced by the fibroblasts: In tumors with an active, unmutated BRCA gene, fibroblasts generated tough fibers forming a dense meshwork, whereas in tumors lacking BRCA activity, fibroblasts produced branching and softer fibers.
These findings suggest that in pancreatic tumors harboring BRCA mutations, the action of fibroblasts as producers of fibers for the intercellular environment is significantly reduced. Instead, they act as immune response suppressors, thus contributing to the malignant tumors’ development.
Blazing the trail for new therapies
All the cells of our body operate in accordance with the same genetic code, but this code is expressed in an utterly different manner in different cell types, tissues and organs. The differences between the body’s various cells are due to changes and additions of modifications to the DNA. The modifications function as punctuation marks enabling the cell to read the code correctly and produce proteins accordingly. These marks determine, for example, whether to increase production of a certain protein (perhaps similar to the role of an exclamation mark) or whether to even read a certain gene at all. Such changes and additions to DNA are being studied closely in a field called epigenetics (Greek for “above genetics”).
The differences between the body’s various cells are due to changes and additions of modifications to the DNA. The modifications function as punctuation marks enabling the cell to read the code correctly
In another study by Scherz-Shouval’s lab, the scientists focused on an additional aspect of fibroblast conversion into “bullies”: epigenetic mechanisms employed by cancer cells for the rewiring of fibroblasts. In this study, led by Coral Halperin from Scherz-Shouval’s lab, in collaboration with Dr. Joschka Hey from Prof. Christoph Plass’s lab at the German Cancer Research Center (DKFZ), the team showed, in mouse models of breast cancer, that cancer cells induce epigenetic alterations in normal fibroblasts. As a result, unlike what happens in healthy tissue, these fibroblasts change their expression of certain genes and produce cancer-assisting proteins. The researchers also found a correlation between these changes and an elevation in the levels of a protein called RUNX1: Its production also increased in fibroblasts of cancer patients, and its activation could be responsible for the epigenetic alterations.
“The treatment of cancer has been revolutionized in recent years with the introduction of immunotherapy – drugs that recruit the immune system in a targeted attack on cancer cells,” says Scherz-Shouval. “Hopefully, the knowledge that we and other researchers have gathered – including the identification of immune-response-suppressing fibroblast subtypes, and of the proteins involved in turning fibroblasts into cancer promoters – can be harnessed for developing new drugs. Such drugs, alongside immunotherapeutic treatments, would effectively target not only cancer cells, but also their collaborators.”