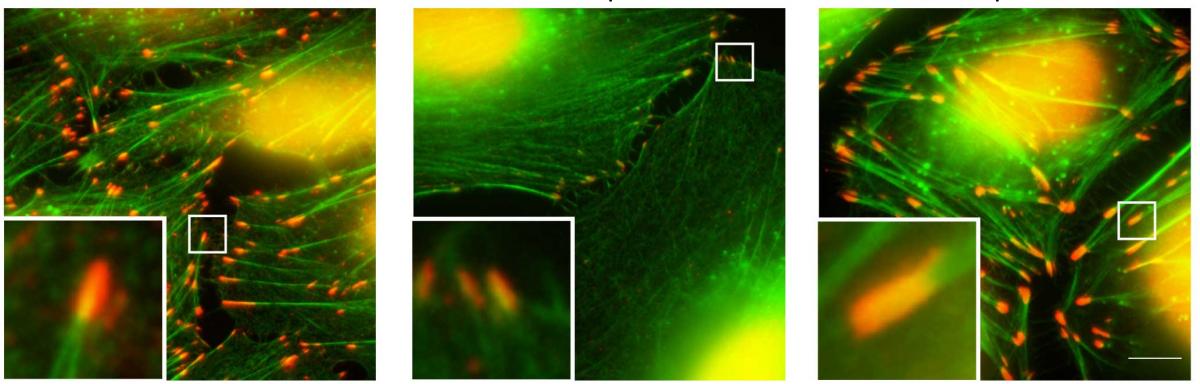
Playing a central role in the reshaping process is a large molecular complex called Arp2/3 that attaches itself to the filaments in the cytoskeleton, causing them to branch out and thereby helping to create the protrusion the cells need for movement. Known for more than two decades and investigated in dozens of laboratories around the world, Arp2/3 has been widely thought to be consistent: the same seven subunits always arranged in the same manner.
Now Weizmann Institute scientists have shown that this complex is modular, and that it can perform different functions by assuming different shapes and attaching itself to different sites in the cytoskeleton. In fact, instead of helping the cell move, it can help it stay in place. In the latter case, the complex contains only three or four core components rather than its classic set of seven, as well as one or two proteins that play a role in cellular adhesion. This hybrid complex attaches to those sites in the cytoskeleton that promote the adhesion. As a result, these sites multiply and increase in size, causing the cell to remain anchored.
Much as the finding was unexpected, the modular mechanism makes evolutionary sense: It’s more economical for the cell to have a single versatile tool that can be adjusted to different uses than to harbor a separate tool for each. The study was performed by graduate student Dror Chorev in the laboratories of
Dr. Michal Sharon of the Biological Chemistry Department and
Prof. Benjamin Geiger of the Molecular Cell Biology Department.
The scientists determined the properties of several Arp2/3 protein complexes, using mass spectrometry. After identifying the presence of hybrid complexes, they confirmed their anchoring effect by manipulating their levels in human cells and comparing the behavior of these cells with the ones containing the better-known seven-unit complex.
The findings open up a new avenue of research by suggesting that in addition to Arp2/3, other important protein complexes might be modular and versatile. They may also one day help control cellular migration on a molecular level, enhancing it or, conversely, blocking it on demand – for example, to prevent the spread of cancer cells throughout the body.
Prof. Benjamin Geiger’s research is supported by the Leona M. and Harry B. Helmsley Charitable Trust; the Adelis Foundation; the Fondazione Henry Krenter; Paul and Tina Gardner, Austin, TX; David and Molly Bloom, Canada; the estate of Anne S. Lubliner; the estate of Raymond Lapon; the estate of Alice Schwarz-Gardos; and the European Research Council. Prof. Geiger is the incumbent of the Professor Erwin Neter Professorial Chair of Cell and Tumor Biology.
Dr. Michal Sharon’s research is supported by the Abramson Family Center for Young Scientists; the European Research Council; and the Sergio Lombroso Award for Cancer Research. Dr. Sharon is the incumbent of the Elaine Blond Career Development Chair in Perpetuity.