Stem cells, like Goldilocks, need a bed that’s “just right” before they can turn into muscle. Too soft, and they become nerve or brain cells; too hard, and they turn to bone. A recent collaboration between scientists in Israel, the US and Germany revealed how the cells “feel” the bed’s hardness and why just the right amount of give in the bed’s “springs” sets them on the path to becoming muscle.
Several years ago, Prof. Dennis E. Discher at the University of Pennsylvania made a surprising discovery: He could direct a stem cell’s fate in the lab simply by adjusting the stiffness of its underlying substrate. A cell sitting on a soft surface not only took on a different shape than a cell on a rigid one; it showed expression of different sets of genes.
Prof. Sam Safran of the Weizmann Institute’s Materials and Interfaces Department, in the Faculty of Chemistry, was intrigued by these results. He wanted to know what it is about stem cells that responds to such purely physical cues as substrate stiffness. Dr. Assaf Zemel, a former postdoctoral fellow in his group, now at the Hebrew University of Jerusalem, and Safran proposed a theory for how the substrate controls the alignment of “stress fibers” within the cell. Discher and his group, including Andre Brown and Dr. Florian Rehfeldt, tested the model experimentally.
The stress fibers are thin but strong strands of the protein actin. These can form a sort of flexible “skeleton” in the cell body, where they are often organized into webs or parallel bundles, endowing the cell with a loosely solid structure, similar in texture to toothpaste. But actin fibers are more than just rigging: They are linked by another molecule called myosin, which hooks onto two parallel actin filaments and uses the energy of the cell to pull on them. This induces a contractile force in the cell. Such “active springs” can be found in many types of cells, where they give rise to an intrinsic tension in the structure; they are also related to the mechanism that enables our muscles to contract.
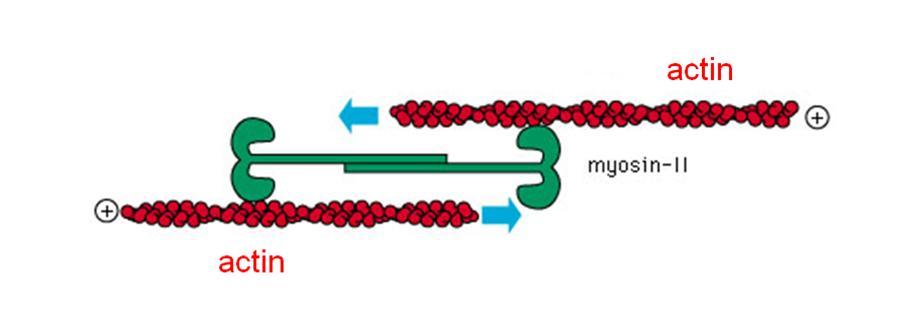
Safran and Zemel reasoned that a living cell, which tends to spread on certain surfaces, attempts to pull itself back by activating its contractile stress fibers. The balance between these two opposing tendencies lies in the relative stiffness of the cell and the substrate. It eventually affects the amount of tension in the cell and the development of the stress fibers themselves. The more rigid the substrate – that is, the less give there is – the harder the fibers pull back. As the stress fibers feel more tension, the physical effort generates a request for reinforcements, and the cell creates additional fibers.
The model predicted that when the amount of give is just right, the stress fibers in the cell will line up in bundles more or less parallel to the long axis of the cell. The experiments, carried out by Discher’s group on synthetic substrates that mimicked the hardness of the different materials encountered by various differentiating stem cells, bore this out.
A paper describing the theory and the experimental findings recently appeared in
Nature Physics.
When the substrate is too soft, the contracting fibers can easily overcome the cell’s stretching. As such a cell is fairly relaxed, it produces relatively few stress fibers, and these pull in no particular direction. When the substrate is stiffer, however, the shape of the cell comes into play. The spread-out stem cell tends to be more oval than round, giving rise to fibers that are longer in one direction. Since longer strands feel more stress than shorter ones, the additional stress fibers develop mainly along the cell’s elongated axis. “And this,” says Safran, “is exactly the arrangement needed to make muscle. Our muscles contract because the fibers all pull together in the same direction.”
If the rigidity of the substrate increases more than this, the fibers become so tense that the cell’s shape and direction cease to be relevant, and new fibers form in every direction.
This physical effect might reach even deeper into the cell, says Safran. Current research in this area is looking into the possibility that the stress fibers pull on the walls of the cell nucleus. In other words, the shape of the nucleus itself may be determined by substrate stiffness, and this could influence which genes are expressed as the cell continues to develop. In related research, together with Discher and his group, Safran and postdoctoral fellow Dr. Benjamin Friedrich are looking closely at how substrate hardness affects the development of the orderly bands of stress fibers found in muscles.
For Safran, this research has had an immediate impact: The close collaboration between the different groups has led a Weizmann graduate, Dr. Amnon Buxboim, to conduct postdoctoral research on stem cell physics in Discher’s lab in Pennsylvania, in collaboration with Safran and Friedrich at Weizmann. Interactions with Zemel in Jerusalem and Rehfeldt, now in Gottingen, Germany, are also ongoing. In the future, the insights from this research might be applied in biomedical research and biotechnology to direct cell and tissue development.
Prof. Samuel Safran’s research is supported by the Carolito Stiftung. Prof. Safran is the incumbent of the Fern and Manfred Steinfeld Professorial Chair.
Protein Reporters Go on Location
Dr. Emmanuel Levy of the Structural Biology Department is working on clarifying the answers to these questions. To do so, he is using a technique he helped develop during his postdoctoral research in the lab of Prof. Stephen Michnick at the University of Montreal. Now, using this technique, Levy, together with Michnick, has observed the comings and goings of the proteins in some 4,000 strains of yeast cells. Their results paint a picture of the cell’s world in which “social networks” and chance meetings seem to play a much larger role than previously thought. The results of their study appeared recently in Cell Reports.
The new technique involves splitting a protein that is vital for the cell’s survival into two halves and attaching each half to another protein. One of those proteins is the reporter, the other the target. If the target protein approaches the reporter, the two halves of the split protein will reunite and the yeast strain will survive and grow. The more abundant the target protein is in the vicinity of the reporter, the healthier the growth of the yeast. The method is so good at measuring protein levels and their localization that the researchers obtained an accurate “census” of nearly all the proteins probed. This, says Levy, means that, for the first time, we can measure with great accuracy the protein concentration in a particular region of a living cell. Measuring local protein concentration is indeed hard-to-impossible to accomplish by other methods – for example, mass spectrometry, which involves killing the cell, or microscopy, which would be a very tedious undertaking on such a scale.
“If we think of the campus of the Weizmann Institute as a cell and the people who work here as the proteins, the reporters we use can record how much time each individual spends in which building,” he says. Their findings show that proteins are highly varied in their habits. Indeed, the scientists were surprised at the wide range they measured: Unlike your average human workers, whose hours on the job don’t vary too much from one to the other, some proteins were thousands of times more industrious – that is, abundant – than others. “It’s as if some go to work for just a minute, while others spend a whole week straight in their workplace,” says Levy. The most abundant proteins were also likely to be seen outside of their regular workplace. Going back to the Weizmann Institute metaphor, a person who works in the cafeteria, if he is there for just a minute a week, will not make an impression. In contrast, someone who actually works in a chemistry lab but also spends a few hours a week in the cafeteria could create more personal ties with the serving staff. This has profound consequences for the incidence of protein interactions. An analysis of the data revealed that, in fact, the chances of any two proteins meeting were first and foremost a product of their numbers in the cell.
Setting up an Experimental Lab