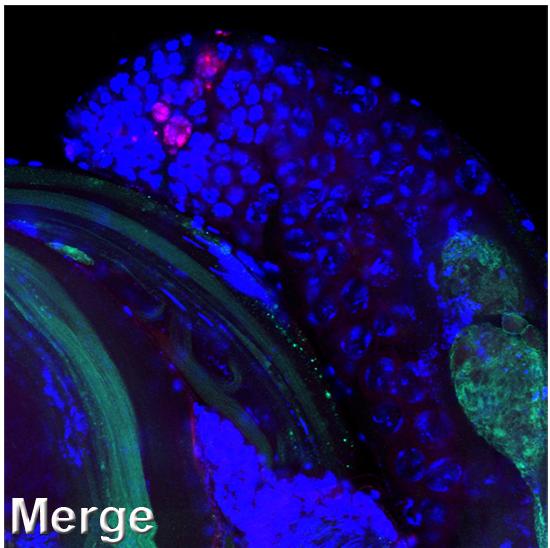
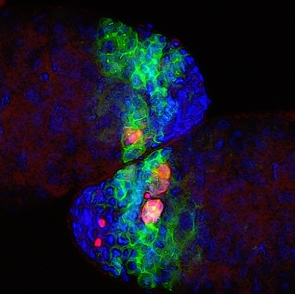
First of all, the scientists confirmed that these germ cells do not die by apoptosis, the most common type of cell death. In particular, they showed that effector caspases, the destructive enzymes that execute apoptotic death, are not involved in germ cell death. Next, they defined the structural hallmarks of germ cell death. Like in apoptosis, the entire cell and its nucleus shrink, and the DNA becomes fragmented. However, many typical apoptotic features are missing in dying germ cells; moreover, unlike in apoptosis, these cells contain large degrading regions and their mitochondria, the energy-producing organelles, become distorted.
The researchers discovered that a central role in germ cell death is played by the mitochondria: These organelles activate a particular gene, htrA2, which makes a destructive protease enzyme. HtrA2 has an equivalent in organisms ranging from bacteria to mammals, which suggests that the findings of the Weizmann fruit fly study are applicable to humans. Yet another major component of the germ cell death mechanism is the lysosome, the cell’s stomach-like organelle that is filled with enzymes for breaking down cellular waste and debris. Lysosomes contribute to cellular destruction by spilling out their contents.
Why do the cells need an alternative death pathway? One possible explanation is that germ cell death is the more ancient in evolutionary terms, while apoptosis, as well as the involvement of caspases in apoptotic death, may have evolved more recently. That could be why flowers and yeast lack conventional caspases and make use of a cell death pathway that is similar to germ cell death, whereas cell death is not the only function of the caspases in multicellular animals.
Knowing the mechanism of germ cell death might have important implications for cancer research, among other things. It may, for instance, help explain the origins of testicular cancer, in which germ cells in the testes multiply uncontrollably. On a more general level, it may help in the development of anti-cancer drugs that could kill cells by an entirely new mechanism, helping to overcome the drug resistance that often emerges in response to more conventional chemotherapy.
Dr. Eli Arama’s research is supported by the Yeda-Sela Center for Basic Research; the Fritz Thyssen Stiftung; and the late Rudolfine Steindling. Dr. Arama is the incumbent of the Corinne S. Koshland Career Development Chair in Perpetuity.