When diets fail, people often blame their genes. Now scientists lend weight to this notion, showing that genes may indeed play a role in weight gain in older people. In a new study, Weizmann Institute of Science researchers found that a gene that regulates calcium in cells might contribute to a common phenomenon in the elderly: the frustrating rise in fat and corresponding loss of muscle that often occurs with age, even when people make no changes in their diet.
Calcium is best known as the main mineral in our bones. But it is also stored in specialized cellular compartments in our body, where it acts as a biological “messenger” that conveys information between cells. To maintain this function, a cell must replenish its calcium reservoirs every time the mineral is released. A two-unit system within cells regulates the replenishing: the first is a sensor that monitors the amount of calcium in stock; the second is a one-way channel in the cell’s membrane that allows calcium to pass through. The channel is normally closed to traffic, only opening to allow the passage of calcium ions when the sensor detects a depletion of the reservoirs.

Scientists from Prof. Eitan Reuveny’s research group at Weizmann’s Biomolecular Sciences and Molecular Neuroscience Departments thought that this regulatory mechanism – which keeps the cells’ calcium levels within the proper range – could be involved in the metabolic changes that take place in the human body as it ages. The researchers had begun to study the mechanism back in 2012, when they discovered a protein named SARAF that can limit calcium entry into the cell by locking on to the calcium sensor when the reservoirs are full, cutting off its communication with the channel.
""When we put the genetically altered obese mice on a strict training program, they successfully lost weight"
In the new study, led by Dr. Diana Gataulin from Reuveny’s research group, the scientists pursued the broader regulatory role they thought this protein might play in the body. To this end, they compared regular mice with genetically engineered ones in which the gene for SARAF production had been deleted.
The most visible effect of the deletion was the slowing of the mice’s metabolism and the gain in body fat. At the age of three months, these mice already weighed 10 percent more than the unaltered ones. The slowdown in metabolism worsened over time, so that at the age of one, the mice lacking SARAF gene activity weighed 20 percent more than the control mice.
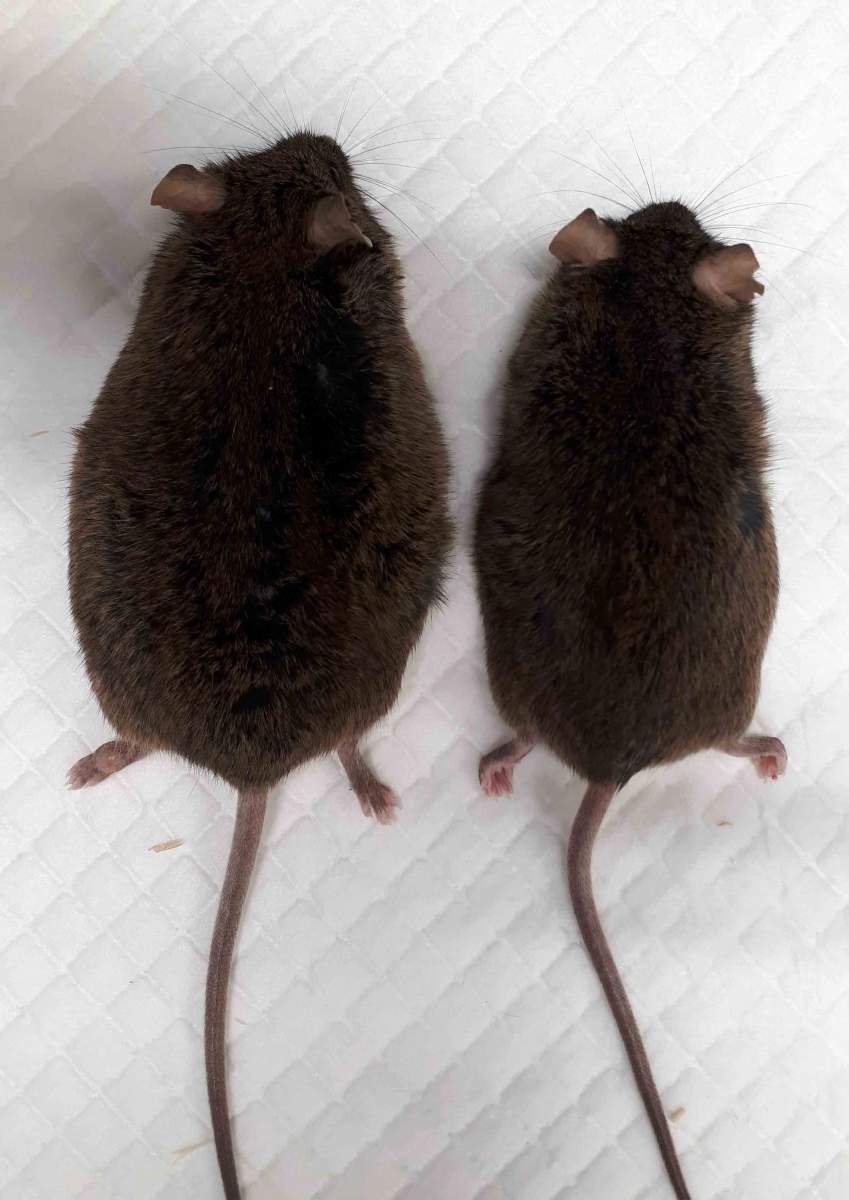
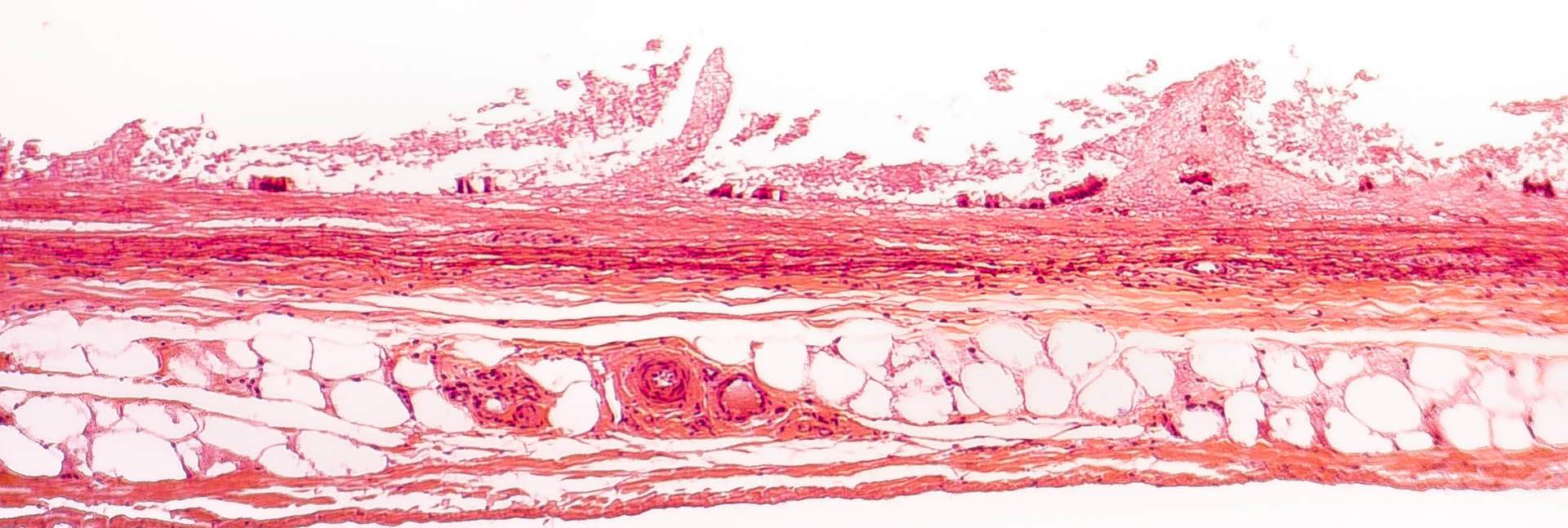
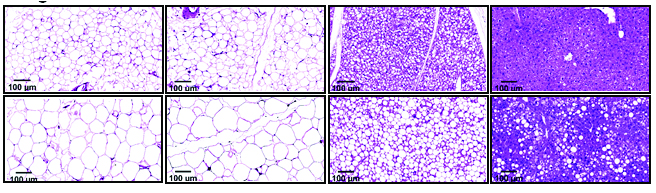
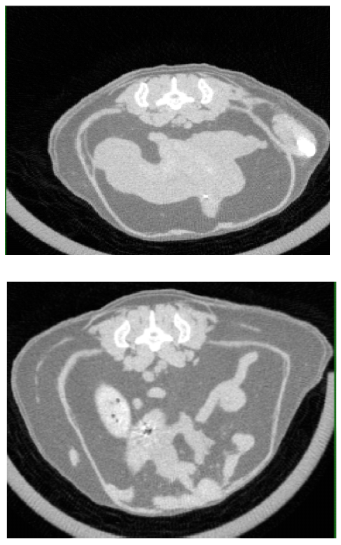
Mice are nocturnal animals, extremely active in dim light conditions, but the obese mice moved less than normal during nighttime. Their tissue composition gradually changed, more of their bodies turning to fat, less to muscle. A CT scan showed that the fat accumulated primarily in the abdomen – similar to the way fat tends to accumulate in older people – and the individual fat droplets inside cells grew significantly. The researchers also found that mice lacking the SARAF gene had higher levels of white fat, the type related to obesity, which accumulated at the expense of brown fat, the type that is beneficial to healthy metabolism and heat production.
By the time the mice turned one (older-middle-aged for a mouse), white fat had also accumulated in their livers, causing fatty liver disease. This disease increases the risk of liver cirrhosis and cancer. These mice also suffered from hypothyroidism, which slows down metabolism and contributes to obesity. The genetically engineered mice underwent all of these changes despite eating the same diet and maintaining the same blood sugar levels as the control mice.
Next, the scientists explored the potential mechanism by which SARAF affects metabolism. They first focused on the hormone vasopressin, which is secreted by the pituitary gland in response to a signal from the brain that the body needs an energy boost. Vasopressin quickly triggers a release of calcium messengers from the cellular reservoirs, telling the body to accelerate energy production. When the scientists added vasopressin to mouse liver cells lacking SARAF, calcium was released more slowly than it should have been. This meant that instead of peaking when the body needed an influx of energy, calcium in those cells reached its highest levels much later. In other words, liver cells took a long time to respond to the vasopressin signal, ultimately producing too much energy, too late.
Since unused energy is stored as fat, these findings reveal a possible molecular mechanism behind what’s known as sarcopenic obesity – a term that describes age-related weight gain that is unrelated to diet and is accompanied by muscle loss and a disproportionate increase in body fat. “Our findings suggest that impaired SARAF activity is a possible cause of sarcopenic obesity,” Gataulin says.
“Obesity is a global epidemic that poses a health risk to hundreds of millions of people,” says Reuveny. “Discovering a gene with key involvement in age-related obesity paves the way for developing medical treatments for this condition.”
Reuveny notes, however, that the effect of genes on age-related weight gain is not set in stone. “When we put the genetically altered obese mice on a strict training program, they successfully lost weight,” he says. “Physical activity may be especially important as people age, in light of the genetic predisposition to obesity that can develop later in life.”