The multipronged attack encompasses studies in cell differentiation, growth regulation, genetics, molecular therapeutics, and diagnostic approaches. Geneticists, biophysicists, immunologists, and cell biologists collaborate in this interdisciplinary effort.
Among their seminal achievements is the identification of the genetic origins of some types of leukemia as well as the discovery of genes that induce or suppress malignancies. Institute scientists also were among the first to demonstrate that cancer development is a multistage process and that certain cancerous cells can be made to revert to normal.
Today's research includes magnetic resonance spectroscopy to detect and help treat breast cancer, photodynamic techniques for attacking melanomas, development of a lung cancer vaccine, a bone marrow transplant technique for treating leukemia, as well as projects which have a bearing on the treatment of ovarian cancer and brain tumors.
Tumors need a blood supply
Weizmann Institute scientists confirmed that only after new blood vessels develop and begin to carry oxygen and nutrients to a malignant tumor, can this tumor develop and grow rapidly. It was clear from this research that a new malignant tumor that is not accompanied by blood vessel development cannot grow larger than one millimeter in diameter. At this stage, tumors usually do not behave aggressively against the body. For research purposes the scientists developed a new system to monitor blood vessel growth and function via magnetic resonance imaging (MRI). This system was applied in order to better understand how oxygen deficiency, hormonal conditions and surgical wounds alter the recruitment of blood vessels and induce tumor growth.
Breast cancer: Tamoxifen's weak spot
Weizmann Institute scientists discovered a major way in which tamoxifen, used to treat breast cancer, halts the development of malignant tumors. The scientists discovered that this drug, which blocks the activity of estrogens (the female sex hormones), inhibits the growth of new blood vessels - and thereby deprives the tumor cells of nutrients and oxygen and "starves" them to death.
Institute researchers monitored the process via magnetic resonance imaging (MRI) and discovered that following treatment with tamoxifen, blood vessels are diminished in the area of the malignant tumor, halting its development and causing the tumor cells to die. However, after a time, new blood vessels develop near the dead regions as a result of the stress conditions. The scientists showed that the regrowth of blood vessels due to stress is stimulated by various mechanisms that are independent of estrogen activity and is therefore not influenced by tamoxifen.
Reducing breast cancer biopsies and improving diagnosis
Weizmann Institute scientists developed a noninvasive method to distinguish between cancerous tumors (of the breast) and benign lumps. The method is based on magnetic resonance imaging (MRI) and it should decrease the need for invasive biopsy examinations and help doctors better understand how well a drug treatment is working.
The method is based on injecting a harmless contrast material into the bloodstream of the patient and tracing it via MRI. The different rate and manner in which the contrast material penetrates malignant and benign growths can be distinguished by the highly-sensitive MRI protocol and analysis developed by the Weizmann scientists. The MRI scans then highlight these differences, enabling doctors to distinguish between malignant and benign growths.
In addition, the method supplies information on the development of microscopic blood vessels in the area of the tumor, which should help forecast its development rate. New blood vessels are essential for the development of cancerous growths because they carry oxygen and nutrients to the tumor cells. By spotting the earliest, tiniest new capillaries, the MRI system can identify a potentially aggressive tumor.
The method, termed the Three Time Point (3TP method), also enables monitoring of the size of the spaces between cells in a tumor. This serves to identify the type of growth and should assist in evaluating the effectiveness of drug treatments. For example, a reduction in the number of blood vessels and a widening of the intercellular spaces (which means there has been a decrease in the density of cells in the area), may indicate that a drug treatment has been successful in stopping tumor growth.
Turn down the amplifier
Weizmann Institute scientists discovered the function of a molecular amplifier that strengthens the chemical signals that cause cells to become cancerous. This amplifier is an enzyme found in large quantities on cancerous cell membranes (especially in breast, ovary and lung tumors). Institute researchers demonstrated that the enzyme, called ErbB-2, collaborates with other proteins to accelerate the chemical signal that instructs a cell to divide - a process that is thought to lead to cancerous growth. This finding may serve as a foundation for a new cancer treatment based on "silencing" this molecular amplifier.
Vaccine that may stop the spread of cancer
Weizmann Institute scientists took a significant step toward the development of a vaccine against certain types of cancer during research that aimed to stop the formation of metastases - the dangerous step during which a single tumor begins to spread throughout the body.
The starting point was to understand the process by which the immune system's T lymphocytes (a type of white blood cell known as a T cell) are supposed to identify and destroy cancerous cells being swept along in the blood stream on their way to establish new metastases. Sometimes the immune system cells fail to identify cancerous cells. It became clear that this is because the cancer cells have a mechanism for appearing to be the same as healthy cells.
However, cancer cells can be distinguished from healthy cells if you know where to look - specifically, at fragments of proteins originating within the cell that are exhibited on the cell membrane. Some of these fragments in cancer cells differ from their parallel fragments in healthy cells (often as a result of a genetic mutation). Weizmann researchers discovered such a protein fragment, isolated it from lung cancer cells, and used it successfully as a vaccination preparation. This enabled the immune system to learn to identify cancer cells and, when the T cells came into contact with the mutant proteins - even at very low concentrations - they attacked and destroyed the cells.
Another team of Weizmann Institute researchers have invented a way to produce killer T cells outside the body. This involved creating monoclonal antibodies (identical antibodies from identical cells) directed against specific components of cancerous growths. In this technique, the antibodies lead the T cells to their targets. It is hoped that this method may be developed further to treat cancers of the ovaries, breast and colon.
Cancer under pressure
Weizmann Institute of Science researchers are developing a new technology by which cells taken from cancerous growths will be subjected to hydrostatic pressure and then treated with a natural fixative to retain the effect of the pressure. The treated cancer cells appear to be able to serve as a basis for an anticancer therapy. The method is currently undergoing clinical trials.
Using light and chlorophyll to destroy tumors
A team of Weizmann Institute scientists is developing an innovative photodynamic approach to destroy tumors. This technique is based on a non-toxic chemotherapy drug, a water soluble derivative of the green plant pigment chlorophyll, that is injected into the blood stream or directly into the tumor. When the drug is illuminated by light in a controlled fashion in the tumor it becomes toxic, destroying tumor blood vessels and cells while having a minimal effect on healthy tissue.
This new "green" photodynamic material was found to be efficient in curing melanoma with a success rate of 85 percent. It clears faster from the body than the materials used in standard photodynamic therapy, which tend to leave patients with a heightened sensitivity to strong light. This technique is being developed for future therapeutic applications through Yeda.
Signs of a nervous disorder
Weizmann Institute scientists have isolated a protein called MAP2d that may help in the early diagnosis of neuroblastoma, a common childhood cancer that originates in the sympathetic nervous system which controls autonomous body functions like the heart beat and digestion. The researchers, using chemical and immunological methods, discovered that MAP2d occurs in embryonic human brain and neuroblastoma tumors. MAP2d is clearly different from similar proteins found in other types of cancer growth or in human adult brains. The presence of this protein may therefore be useful in early diagnosis of the disease.
MAP2d is connected to microtubules, which are an important component of the cell's structure, or cytoskeleton. Microtubules supervise many cell functions, including nerve impulses, secretion and chromosome separation during cell division.
Out of control
Researchers at the Weizmann Institute of Science demonstrated that cancer cells are characterized by an inability to react correctly to chemical messages directing them to stop growing and proliferating. The researchers found a new group of genes responsible for producing various proteins that play an important role in the cell's reaction to these chemical messages. They discovered that a flaw in these genes makes the cell unable to react correctly to the chemical messages, which leads to an uncontrolled growth and proliferation of cells, for example, in a malignant tumor. The knowledge obtained in these studies may assist in developing ways to strengthen the control of cell proliferation, resulting in the ability to halt the growth of malignancies.
Genes that cause leukemia
Weizmann Institute scientists have discovered two genes (ABL and BCR) on chromosomes 9 and 22. Under certain circumstances they combine to produce a gene which encodes a fused protein that leads to the development of the common cancer, chronic myeloid leukemia. This study showed for the first time that the union of two proteins can be a key factor in the development of cancer.
The same research team identified several genetic partnerships between the ALL-1 gene, located on chromosome 11, and a series of genes located on other chromosomes. All such partnerships lead to the synthesis of a new protein whose composition is dictated by the two genes involved. These proteins lead directly to acute leukemia, in particular in infants and in some patients who have undergone treatment with anticancer drugs. Weizmann scientists are continuing their efforts to understand the mode of activity of the protein encoded by the normal ALL-1 gene and the function of the cancer-inducing proteins formed as a result of the union of ALL-1 with other genes.
Another team of Institute researchers discovered that the gene AML-1, located on chromosome 21 and known to be involved in leukemia, may also induce this, mostly in sufferers of Down's syndrome. This syndrome emanates from the existence of a third (and unnecessary) copy of chromosome 21 in each of the body's cells. The scientists discovered that the third copy of the gene AML-1 causes the formation of a protein that may act as a dominant negative and be responsible for myeloid leukemia. Different defects in this gene appear to produce leukemia in people without Down's syndrome. These discoveries should help in understanding the leukemic process and in devising ways to cure myeloid leukemia.
Growth, development and cancer
In research carried out with fruit flies, Weizmann Institute scientists discovered a phenomenon that could help to understand and inhibit cancer in humans. The scientists identified a fruit fly gene that plays a role both in embryonic development and perhaps in carcinogenic processes. The gene has been well-preserved throughout evolution, which means that there are only relatively small differences between its manifestation in the fly and in more developed animals, including humans. Understanding processes in which this gene is involved may lead to new methods for treating breast cancer and other diseases, as well as providing a better understanding of how organs develop in an embryo.
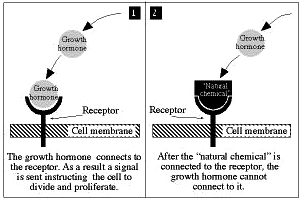
The scientists examined the process by which the growth hormone EGF binds to a specific protein receptor on the cell membrane. This binding triggers a biochemical process that causes the cell to divide and multiply, or to specialize in a specific field (to become a nerve, muscle, blood cell and so on). When this vital process unfolds without appropriate control, proliferation of undesirable cells may occur, resulting in malignancy.
Weizmann Institute scientists discovered that the body controls the communication between EGF and the receptor by changing the rate of production of a natural chemical which prevents this connection. As more growth hormone molecules connect to more receptors, the body produces more of the chemical. The overall effect is to maintain the appropriate quota for connections between molecules of growth hormone and receptors. The gene responsible for production of this chemical has been identified and its role in the system has been understood and explained for the first time.
This research may bring about the development of drugs which will replace the missing natural chemical or neutralize an excess of growth hormone. These drugs may prevent uncontrolled cell proliferation processes, and thereby stop the progress of cancer.
Multistage theory of cancer
In the 1950s, Weizmann Institute scientists were among the first to demonstrate that certain types of cancerous growths develop in a two-stage process and that cancer depends on the presence of multiple factors. Until then, it was believed that one factor was sufficient for cancer to develop. This discovery improved the understanding of various scenarios that might lead to malignancy. It also provided an explanation for the time lapse (sometimes several decades) between exposure to carcinogenic substances and the development of a tumor.
Malignant cells back on the right track
Weizmann Institute scientists were the first to show (in the 1960s) that cancer cells- in tissue cultures or in living organisms- can be made to revert to normal and healthy behavior. This finding led to the development of "differentiation therapy" for treating cancer through restoring the cells' capability to grow and develop in a benign and orderly fashion.
Proteins that track the origins of a tumor
Weizmann Institute scientists developed a battery of monoclonal antibodies (a highly uniform population of antibodies) that specifically bind to components of the cytoskeleton. Antibodies to one specific family of such filaments (known as intermediate filaments) can distinguish between cells derived from different tissues. By precisely defining a particular class of these protein filaments in malignant tumors, it is possible to determine their origin. This approach allows pathologists to decipher biopsy findings, especially when regular approaches are not conclusive, which in turn may help physicians in selecting the most appropriate anticancer treatment for a patient.
The key to tumor suppression: p53
Today it is clear that genetic defects play a central role in the mechanisms responsible for the cell changes that lead to malignancy. The multidisciplined offensive on cancer at the Weizmann Institute began with attempts to understand the part that some genes play in cell differentiation and in controlling cell proliferation, as well as to identify the genetic defects that can result in the development of cancer.
The genes involved in the development of malignant diseases can be divided into two main groups: genes whose activity and expression cause cancer, and those whose manifestation and normal activity suppress the processes that can lead to cancerous tumors. When a problem occurs in a tumor-suppressing gene, it fails to act properly, perhaps opening the way for malignant disease.
Weizmann Institute scientists cloned, for the first time, the tumor-suppressing gene p53, which is associated with about half the malignant tumors in humans. The scientists played an important part in elucidating the role of this gene. Later it became clear that the protein that is a product of this gene identifies defects throughout a cell's genes (for example, as a result of radiation damage or other environmental factors, and also any errors that may creep in when genes are replicated during cell division). The p53 protein product can assess the damage and decide whether to repair it and in so doing, enable the cell to continue living or, if the damage is too great, to cause the cell's destruction.
Weizmann scientists demonstrated that p53 is associated with the completion of the cell differentiation process and the induction of the death program of the cells, called apoptosis ("shedding" or "falling" in Greek), to emphasize the similarity between the phenomenon and the process of leaves dying in autumn. Hidden in each of the body's cells is a unique genetic suicide program which, when activated, causes the cells to commit suicide.
This ability to instruct cells to die is essential to the body's normal activity. Loss of this capability - for example, as a result of a mutation in p53 - may lead to a proliferation of cells carrying a defective genetic make-up, sometimes creating a malignant tumor. A defective p53 gene may also abort the differentiation process, allowing the cell to continue past its intended goal.
Institute scientists continue to contribute to research into p53 in the hope of developing genetic techniques to repair or compensate for failures in its functioning. These techniques should help in inhibiting a significant number of cancers in humans.