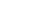- How genes control the immune system
- Whose bone marrow is it?
- Bringing wayward cells to task
- How cells are induced to commit suicide
- Joint action
- Drawing the fire of a misguided immune system
- Pioneers in producing homogeneous antibodies
- How an antibody makes contact
- An artificial antibody
- Guided missiles that seek out cancer cells
- Putting interferons and other cytokines to work
- A control for the immune system
How genes control the immune system
In the 1960s, Weizmann Institute scientists were the first to show how genes control the ability of the immune system to react to specific foreign agents. The method was revealed by using relatively simple synthetic antigens to gain control of the immune reactions in varieties of inbred mice, thereby demonstrating and examining the changes taking place in immune reactions as a result of certain genetic changes.
Whose bone marrow is it?
Weizmann Institute scientists developed a method that allows bone marrow cells to be transplanted into leukemia patients from noncompatible donors - greatly increasing the possibility of effective treatment. Until this breakthrough in 1993, this type of transplantation was considered impossible because the recipient's immune system would reject the foreign cells.
Leukemia is a form of cancer, recognizable by overproduction of any one of the different types of white blood cells and suppression of other blood cells. Since all blood cells are created from the stem cells found in bone marrow, doctors and scientists assume that the illness emanates from irregular functioning of the stem cells. In order to repair this flaw, doctors destroy (via radiation and chemotherapy) the patient's bone marrow and then replace it with a transplant of healthy bone marrow cells.
The problem is that the patient's immune system rejects the foreign cells if they are not compatible, so Weizmann scientists proposed transplanting megadoses of bone marrow to overcome the immune system's rejection.
However, the quantity of bone marrow that can be taken from a donor is rather limited. Therefore, the researchers increased the amount of stem cells available for transplant by collecting them from the peripheral blood circulation. This involves increasing (via a hormonal preparation) the quantity of stem cells in the blood and then separating them for transplant.
Bringing wayward cells to task
Autoimmune diseases occur when the body's immune system, meant to protect it from foreign invaders and infections, turns against the body's own healthy tissues or substances vital for its well-being, and attacks them. Among the diseases included are: multiple sclerosis, juvenile diabetes, rheumatoid arthritis and systemic lupus erythematosus.
In the 1980s, Weizmann Institute scientists proposed a method to grow these wayward immune cells on tissue cultures and to use them in preparations that may serve as a vaccine against these diseases. The technique is called T cell vaccination and is undergoing clinical trials in several countries.
How cells are induced to commit suicide
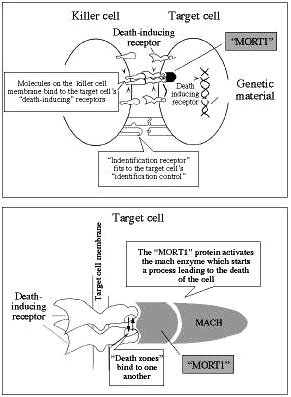
Weizmann Institute researchers were the first to propose that immune system cells (a type of white blood cell called killer T lymphocytes) that kill tumor cells, carry out their mission by triggering death-inducing receptors on the surface membrane of their targets. This induced "suicide" is based on an internal genetic program embedded in every cell of the body (including the T cells themselves), which instructs them to die- and in death to bequeath life to the body. This self-destruction is called apoptosis ("shedding" or "falling" in Greek), to emphasize the similarity between the phenomenon and the process of leaves dying in autumn.
Other Weizmann researchers discovered the biochemical components of the chain by which the cells commit suicide and showed it is much shorter than was formerly believed. The chain begins at a receptor on the cell membrane that receives various chemical messages from outside. A specific region of the receptor, sunk into the target cell, binds and activates a protein (called MORT1) that binds to and activates an enzyme (called MACH) which kills the cell by rupturing specific essential proteins. Weizmann Institute investigators discovered and cloned the genes responsible for production of the MORT1 protein and the MACH enzyme.
These studies of the processes of cell death may lead to various manipulations that will strengthen or weaken the immune system's ability to fight cells conquered by bacterial or viral infection, cancerous cells or cells that the body has mistaken for "enemies" (which can cause autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and juvenile diabetes).
Joint action
Weizmann Institute scientists discovered that antibodies from the gamma globulin family may accelerate the growth of sodium salt crystals of uric acid (monosodium urate) in the joints of patients suffering from gout. They achieved this by extracting gamma globulin antibodies from the joint fluid of gout patients and showed, in a test tube, that these antibodies can accelerate the formation and deposit of the crystals. This process may be a factor in the development of the disease.
In this instance, gamma globulin appears to operate differently from most types of antibodies: When it binds to a specific target, it doesn't destroy it. Rather, the opposite occurs: It helps it to grow and develop. The researchers suggested that this was because of special properties of the binding site.
This research may assist in understanding how antibodies identify foreign targets, and also in comprehending the operational mechanism of gout and other diseases associated with the formation of crystals in the body.
Drawing the fire of a misguided immune system
The disease myasthenia gravis is expressed by significant muscle weakness emanating from a disturbance in the transfer of signals from nerves to muscles. Weizmann Institute scientists found that those suffering from this disease produce antibodies that attack a specific protein at the communication junctions between the muscles and nerves. Following this, researchers identified the small fragments of this protein that are targeted for attack by the immune system. This finding led to the planning of synthetic imitations of these fragments whose purpose is to "draw the fire" of the immune system and thereby stop it from interfering with the natural substances that are vital to the body's activity. This development may represent an important step toward specific treatment for myasthenia gravis.
Pioneers in producing homogeneous antibodies
Weizmann Institute investigators were among world pioneers in producing monoclonal antibodies (absolutely identical antibodies originating from identical cells) in tissue cultures, bacteria, and animals. They were the first to use this technology in Israel.
Monoclonal antibodies prepared at the Institute were used for basic research and also to develop diagnostic tests related, for example, to pregnancy and cancer. Other monoclonal antibody preparations developed by Weizmann scientists are being used in understanding and inhibiting allergic reactions, in designing new ways of preventing the development of cancerous tumors, and in studies of autoimmune diseases (diseases that occur when the immune system mistakenly attacks healthy body tissue and substances essential for its normal functioning).
Weizmann Institute scientists also pioneered the production of "catalytic antibodies." These are special antibodies which can perform and enhance very specific chemical reactions, unlike common antibodies which can only bind and neutralize foreign invaders (antigens). As such, they hold the potential for providing us with new enzymes which are applicable to biotechnology.
How an antibody makes contact
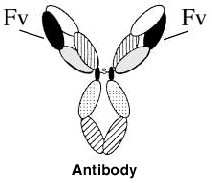
Weizmann scientists identified where and how an antibody binds to its antigen, the substance which provokes the immune response. The binding site is located at the end of the "variable" region of the protein chain in the "arms" of the antibody. (Antibodies are structures composed of several peptide chains that form in the shape of the letter Y. The variable region differs from one antibody to another.) The researchers mapped the area at the binding site that comes in contact with the antigen and built a spatial model of it. This has helped to elucidate the binding process. They also discovered that the antibody is structurally altered after binding to the antigen.
An artificial antibody
Antibodies are produced naturally in the body in white blood cells and Weizmann Institute scientists prepared, for the first time, the smallest fragment of antibody containing all its binding properties. Today, this fragment (called Fv) is used in genetic engineering techniques for producing synthetic antibodies for treating various diseases. This fragment is about one-sixth the size of the whole antibody, which means it can penetrate tissue more easily. Later, using chemical synthesis, they developed the first synthetic antibody in the world.
Guided missiles that seek out cancer cells
Weizmann Institute scientists developed antibodies that selectively bind to specific components on cancer cell membranes. These monoclonal antibodies (absolutely identical antibodies originating from identical cells) can be used to identify and mark cancerous cells and can also act as "guided missiles" that lead anticancer drugs to their target.
In another study, researchers combined anticancer antibodies with the immune system's T-lymphocytes, or T cells, which are supposed to identify, destroy and reject foreign cells (such as an incompatible tissue transplant), cells of the body that have been invaded by viruses or bacteria, and also cancerous cells either primary or on their way to establishing new growths (in a process known as metastases). However, in most circumstances in patients with established tumors, T cells do not identify cancerous cells; they therefore evade capture and can replicate to spread and produce new tumors.
Weizmann scientists used genetic engineering techniques to combine the specificity of the anticancerous antibodies with T cells. These new combinations have proved effective in laboratory experiments and are now being tested in clinical trials in the United States.
Putting interferons and other cytokines to work
Weizmann Institute scientists developed a method to produce the human interferon-beta in laboratory cultures of animal cells. For this purpose, the genetic make-up of hamster cells was modified through the introduction of multiple, activated copies of a human gene responsible for producing interferon-beta. This procedure, upscaled into an industrial process by the locally-established InterPharm biotechnology firm, has an advantage over previous genetic engineering techniques: It yields an interferon-beta with a structure and make-up practically identical to that produced in the human body, thereby greatly reducing side effects when administered to patients.
Interferon-beta functions as a messenger between cells in various tissues, including cells of the immune system, prompting defenses against foreign invaders, especially viruses. Interferon-beta is particularly effective as an inhibitor of tumor growth and as a regulator of immune response. In autoimmune diseases such as multiple sclerosis, interferon-beta has become the approved medication of choice for slowing down the progression of neurological disabilities characteristic of this disease. Additional clinical applications of interferon-beta are for viral diseases such as herpes, papilloma warts, and viral hepatitis, and as an adjuvant in the treatment of advanced colorectal cancer and other malignancies.
In other studies on cytokines, Weizmann scientists pioneered the discovery of interleukin-6, a substance that promotes the formation of new blood cells and regulates the functioning of the liver and of the immune system. By combining the genes encoding interleukin-6 and its specific receptor, Weizmann scientists produced a superactive chimeric protein. For the first time this allows for the laboratory culture of the blood stem cells that are critical for the success of bone marrow transplants, a lifesaving procedure in modern medicine. This chimera, now produced by the abovementioned animal cell genetic engineering, opens up new possibilities for controlling the interleukin-6 system in patients with malignancies and immune system dysfunction.
A control for the immune system
Weizmann Institute scientists isolated a protein that may serve as a foundation for halting autoimmune diseases, diseases caused when the immune system mistakenly attacks healthy tissues in the body or substances vital to its normal functioning. In some of these diseases, the protein interleukin-6 acts like a chemical messenger that arouses the immune system to react offensively. Interleukin-6 has several functions, one of which is its essential role in the life of antibody-producing cells.
The protein isolated by Weizmann scientists (called restrictin-P or activin A) binds to the immune system cells and transmits a message that cancels the "life message" of interleukin-6. Restrictin-P actually seems to kill or halt the reproduction of some antibody-producing cells and also weakens certain immune reactions. This selective inhibition of the immune reaction may be used to develop treatment and prevention methods for diseases of the immune system such as leukemia and autoimmune states.